Chitosan oligosaccharide Schiff base phosphonate as well as preparation method and application thereof
A technology of Schiff base phosphonate and chitosan oligosaccharides, applied in the field of development and utilization of biological resource pesticides, to achieve the effects of easy control of temperature conditions, significant induction of disease resistance, and good resistance to tobacco mosaic virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
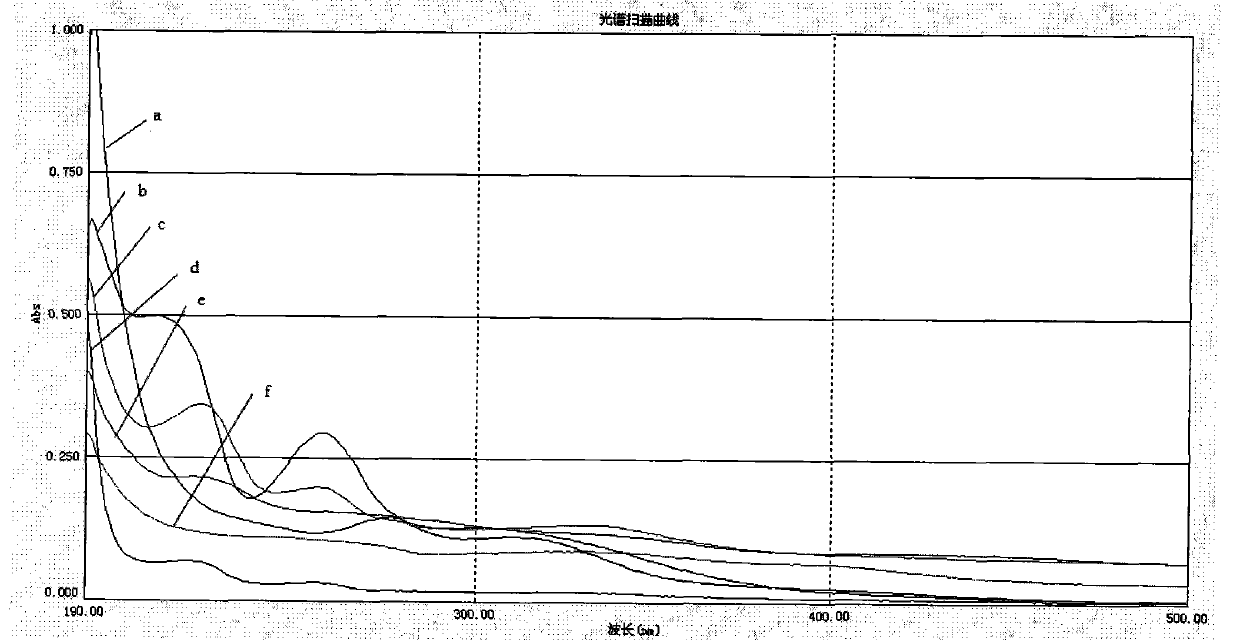
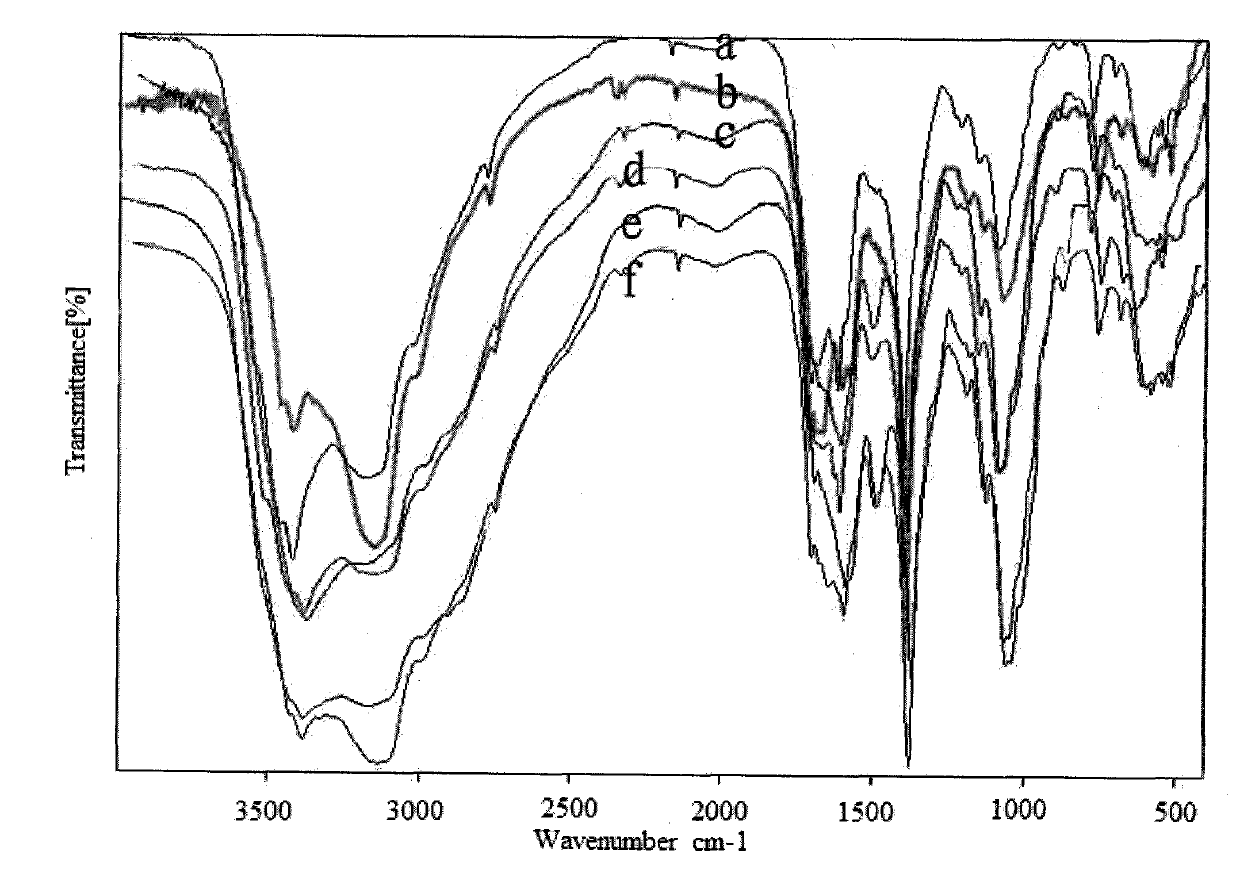
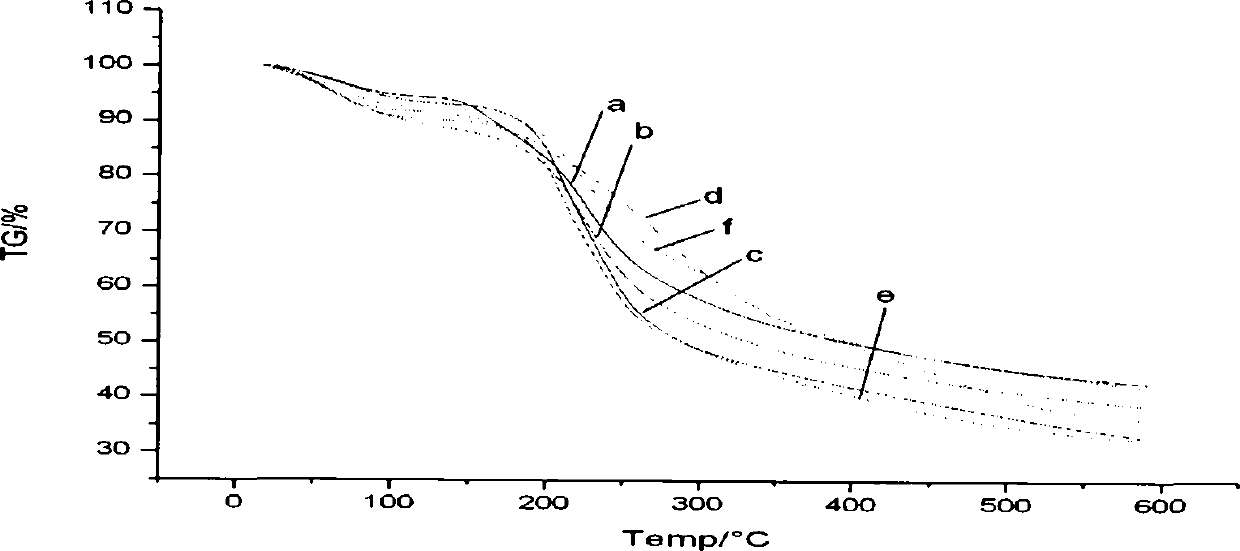
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of 5-chlorosalicylaldehyde chitooligosaccharide Schiff base phosphonate
[0042] (1) Preparation of salicylaldehyde chitooligosaccharide Schiff base
[0043] After soaking and swelling 7.0186g of chitosan oligosaccharide in 20mL of absolute ethanol solvent, 8.0538g of salicylaldehyde and 0.2445g of NaOH were added (to adjust the pH value of the solution to 7.5-9), and microwave heating was performed under reflux for 1.5h. After the reaction, the product was dried by suction filtration, and the product was extracted with ethanol for 24 hours to remove excess salicylaldehyde and substituted salicylaldehyde in the product, and the product was vacuum-dried at a temperature lower than 50°C. Yield 4.2815g.
[0044] (2) Preparation of 5-chlorosalicylaldehyde chitooligosaccharide Schiff base phosphonate (S-COS-P)
[0045] 1.8339 g of the salicylaldehyde chitooligosaccharide Schiff base (S-COS) prepared in the above step (1) was reacted with 2.2766 g of d...
Embodiment 2
[0046] Example 2 Preparation of 5-chlorosalicylaldehyde chitooligosaccharide Schiff base phosphonate
[0047] (1) Preparation of 5-chlorosalicylaldehyde chitooligosaccharide Schiff base
[0048] Soak and swell 6.5852g chitosan oligosaccharide in 50mL absolute ethanol solvent, add 4.6473g 5-chloro salicylaldehyde and 0.9910g NaOH (adjust the pH value of the solution to 7.5-9), microwave heating and reflux reaction for 2h. After the reaction, the product was dried by suction filtration, and the product was extracted with ethanol for 26 hours to remove excess salicylaldehyde and substituted salicylaldehyde in the product, and the product was vacuum-dried at a temperature lower than 50°C. Yield 7.1272g.
[0049] (2) Preparation of 5-chlorosalicylaldehyde chitooligosaccharide Schiff base phosphonate (S-COS-P) 3.2071g
[0050] 2.0484g of the 5-chlorosalicylaldehyde chitooligosaccharide Schiff base (S-COS) prepared in the above step (1) was reacted with 3.2071g of diethyl phosphite...
Embodiment 3
[0051] Example 3 3,5-dichloro salicylaldehyde chitooligosaccharide Schiff base phosphonate
[0052] (1) Preparation of 3,5-dichloro salicylaldehyde chitooligosaccharide Schiff base
[0053] Soak and swell 3.6080g chitosan oligosaccharide in 40mL absolute ethanol solvent, add 3.0732g 3,5-dichlorosalicylaldehyde and 0.2038g NaOH (adjust the pH value of the solution to 7.5-9), microwave heating and reflux reaction for 2.5h. After the reaction, the product was dried by suction filtration, and the product was extracted with ethanol for 27 hours to remove excess salicylaldehyde and substituted salicylaldehyde in the product, and the product was vacuum-dried at a temperature lower than 50°C. Yield 3.756g.
[0054] (2) Preparation of 3,5-dichloro salicylaldehyde chitooligosaccharide Schiff base phosphonate (S-COS-P)
[0055] 1.4653 g of the 3,5-dichloro salicylaldehyde chitooligosaccharide Schiff base (S-COS) prepared in the above step (1) was reacted with 2.7600 g of diethyl phosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com