Drug slow control releaser and preparation method thereof
A technology for controlled release and controlled release of drugs, used in pharmaceutical formulations, drug combinations, drug delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
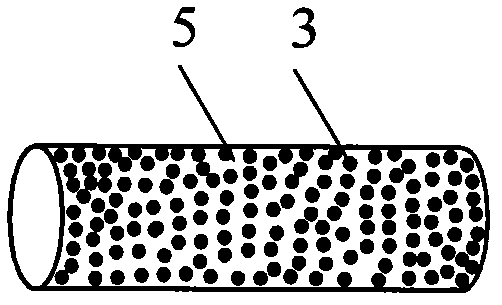
[0027] Embodiment 1: use polycaprolactone capsule tube to prepare the drug delivery body containing levonorgestrel (LNG)
[0028] A preferred embodiment of the drug-containing core in the present invention is that the drug-containing core is composed of levonorgestrel uniformly distributed in polycaprolactone, wherein: the levonorgestrel accounts for about Containing 65% of the weight of the drug core, namely take 400g of levonorgestrel and 200g of polycaprolactone.
[0029] Of course, the content of the components in the drug-containing core can also be: the levonorgestrel accounts for about 70%, or 75%, or 80% of the total weight of the drug-containing core, and the balance is used as the drug-containing core Any one of polylactic acid, polylactide, polylactic-glycolic acid, polylactide-glycolide, polycaprolactone, polyhydroxybutyric acid, polyurethane and silicone rubber in the second polymeric material of the carrier. Thus, drug-containing cores containing levonorgestrel ...
Embodiment 2
[0036] Embodiment 2: the in vitro drug release test that the drug release body that contains levonorgestrel (LNG) is carried out
[0037] The drug release body containing levonorgestrel (LNG) made in Example 1 is put into 100ml distilled water, and in the constant temperature shaker of 37 ± 2 ℃, the agitation speed of 130 rev / min is kept, every certain time Replace release fluid. Use HPLC to measure the concentration of levonorgestrel in the release liquid, the chromatographic measurement conditions are as described in the literature, and the daily average drug release rate can be calculated according to the measurement results. The drug-containing core prepared in Example 1 was used as a control, and the daily average drug release rate was measured and calculated in the same way.
[0038] Such as figure 2 As shown, the release rate of the drug-containing core forms a peak in the first 6 days in vitro, and then the release rate gradually increases. In the first 3 days outs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com