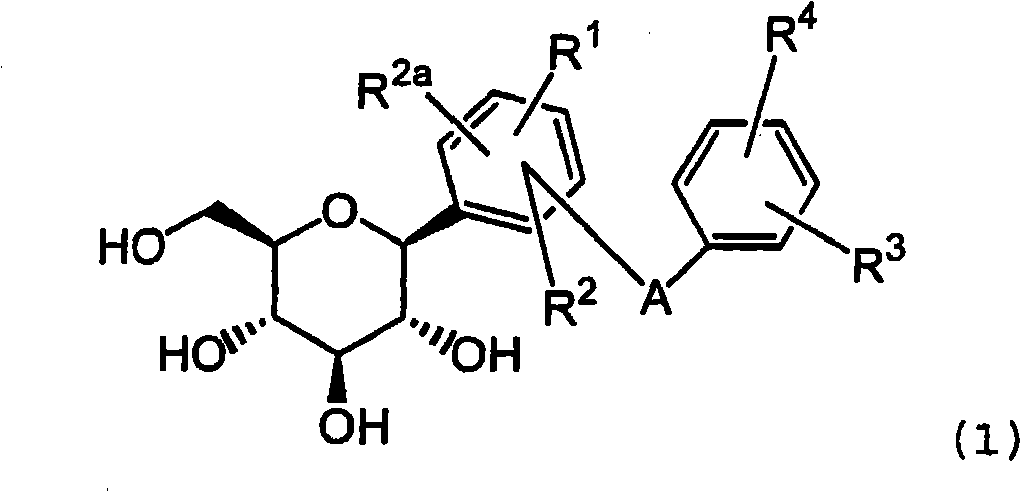
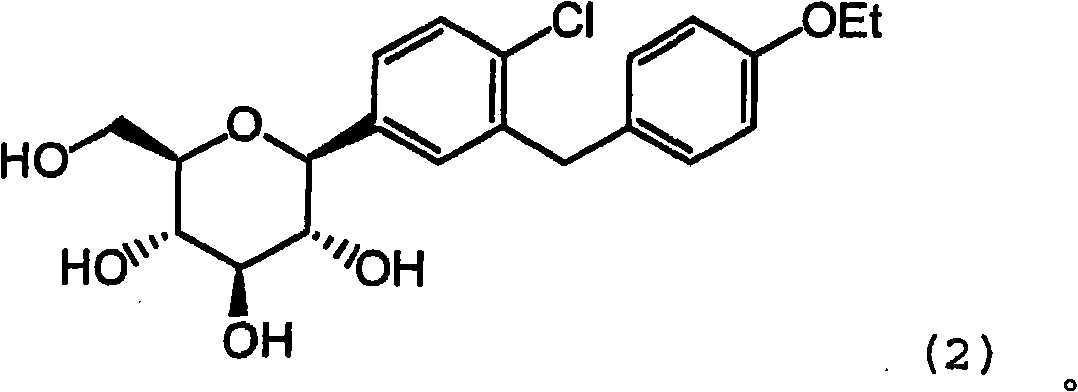
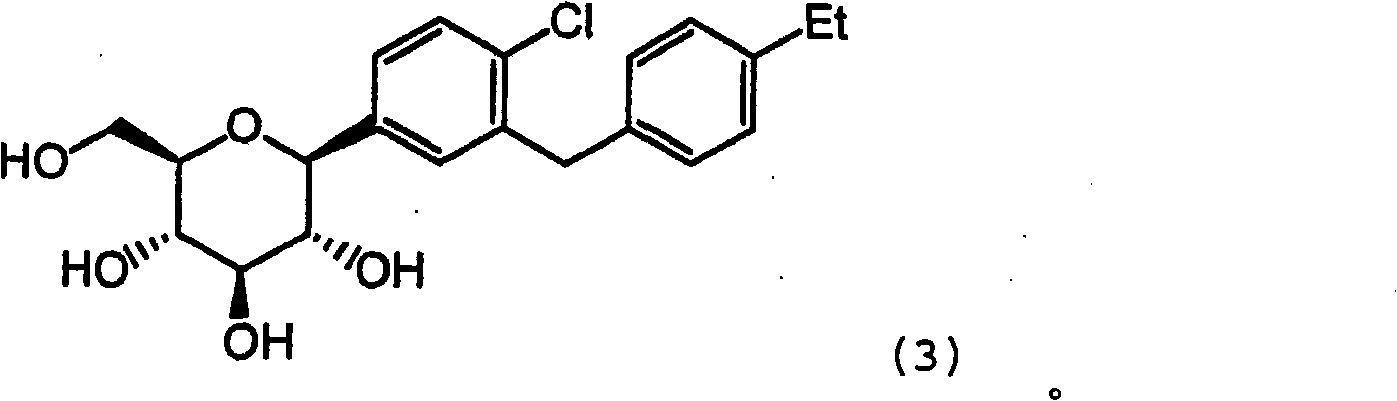
Combination therapy comprising sglt inhibitors and dpp4 inhibitors
A technology for DPP4 and inhibitors, applied in the field of compositions that increase the concentration of active GLP-1 in mammalian plasma, can solve problems such as reducing glucose concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0479] Effect of SGLT inhibitors on plasma active GLP-1 concentrations in glucose-treated DPP4-deficient rats
[0480] (a) Animals:
[0481] DPP4-deficient male Fisher rats (purchased from Charles River Japan, Inc.)
[0482] (b) method:
[0483] (b-1) Preparation and administration of test compound
[0484] The test compound was suspended in a 0.5% carboxymethylcellulose solution containing 0.2% Tween 80 (Tween 80) at the dose shown in Table 1, and was orally administered to the test group at a volume of 5 mL / kg. The vehicle control group received oral administration of 0.5% carboxymethylcellulose solution containing 0.2% Tween 80 (Tween 80) at a volume of 5 mL / kg. Glucose solution (2 g / kg / 5 ml) was orally administered immediately after compound or vehicle administration. (b-2) Blood collection process and determination of active GLP-1 concentration in plasma
[0485] Blood was collected from the tail vein of unanesthetized rats immediately before glucose administration a...
Embodiment 2
[0498] Synergistic Effect of SGLT Inhibitor and DPP4 Inhibitor on Concentration of Active GLP-1 in Plasma of Glucose-Treated Normal Rats
[0499] (a) Animals:
[0500] DPP4-positive male Fisher rats (purchased from Japan SLC, Inc.)
[0501] (b) method:
[0502] (b-1) Preparation and administration of test compound
[0503] Each test compound was suspended in a 0.5% carboxymethylcellulose solution containing 0.2% Tween 80 at the dose shown in Table 2, and was orally administered to the test group at a volume of 5 mL / kg. The vehicle control group received oral administration of 0.5% carboxymethylcellulose solution containing 0.2% Tween 80 (Tween 80) at a volume of 5 mL / kg. Glucose solution (2 g / kg / 5 ml) was orally administered immediately after compound or vehicle administration.
[0504] (b-2) Blood collection process and determination of active GLP-1 concentration in plasma
[0505] Blood was collected from the tail vein of unanesthetized rats immediately before glucose a...
Embodiment 3
[0520] Effects of SGLT inhibitors and DPP4 inhibitors on plasma active GLP-1 concentrations in glucose-treated diabetic mice
[0521] (a) Animals:
[0522] Male BKS.Cg - +Lepr db / +Lepr db / Jc1 mouse; animal model of type 2 diabetes (purchased from CLEA Japan, Inc.)
[0523] (b) method:
[0524] (b-1) Preparation and administration of test compound
[0525] Each test compound was suspended in a 0.5% carboxymethylcellulose solution containing 0.2% Tween 80 at the dose shown in Table 3, and was orally administered to the test group at a volume of 5 mL / kg. The vehicle control group received oral administration of 0.5% carboxymethylcellulose solution containing 0.2% Tween 80 (Tween 80) at a volume of 5 mL / kg. Glucose solution (2 g / kg / 5 ml) was orally administered immediately after compound or vehicle administration.
[0526] (b-2) Blood collection process and determination of active GLP-1 concentration in plasma
[0527] Blood was collected from the tail vein of unanesthet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com