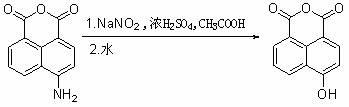Naphthalimide fluorescent dichroic dye containing aromatic ester group and application thereof
A technology of naphthalene imide and aromatic ester, applied in the field of display materials, can solve problems such as restricting application development, and achieve the effects of novel structure, easy availability of raw materials and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
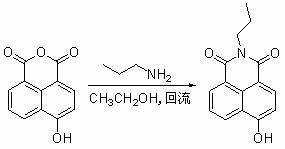
[0025] Synthesis of 4-(p-hexyloxybenzoate)-N-n-propyl-1,8-naphthalimide (N1)
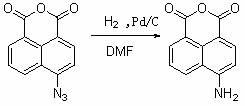
[0026] (1) In a 100mL two-necked flask, add 2.77g 4-bromo-1,8-naphthalene anhydride (10mmol), 40mL DMF, 0.97g NaN 3 (15 mmol), heated to 60° C. for 5 hours, followed by TLC plate to the end of the reaction. Let stand, cool, and slowly pour into 400mL of ice water, a large amount of yellow solid is precipitated, filtered, washed, and dried to obtain 2.27g of yellow solid, yield: 95%.
[0027]
[0028]
[0029] (2) In a 75mL autoclave, add 2g of 4-azido-1,8-naphthalene anhydride (8.4mmol), 0.36g of 10% palladium carbon catalyst, 35mL of DMF, evacuate several times, fill with 1.5MPa hydrogen, and React at 60°C for 4 hours, cool, filter, and wash with hot DMF solution. After the filtrate is cooled to room temperature, pour it into 200mL ice water, a large amount of yellow solid precipitates, filter, wash, and dry to obtain 1.6g of solid, melting point:> 300°C, Yield: 90%.
[0030]
[0031]...
Embodiment 2
[0040] Synthesis of 4-(p-hexyloxybenzoate)-N-n-butyl-1,8-naphthalimide (N2)
[0041] Except that n-butylamine was used instead of n-propylamine, other synthesis and purification methods were the same as in Example 1 to obtain the target product N2 with a yield of 44% and a melting point of 146.5-147.4°C.
[0042] 1 H-NMR (400MHz, CDCl 3 ), δH (ppm): 8.67 (d, J=8.0Hz, 2H), 8.33 (d, J=8.4Hz, 1H), 8.28 (d, J=8.8Hz, 2H), 7.77 (dd, J1=J2 =8.0Hz, 1H), 7.68 (d, J=8.0Hz, 1H), 7.07 (d, J=8.8Hz, 2H), 4.20 (t, J1=J2=7.6Hz, 2H), 4.09 (t, J1 =J2=6.4Hz, 2H), 1.85 (m, 2H), 1.73 (m, 2H), 1.43 (m, 8H), 0.99 (t, J1=J2=7.2Hz, 3H), 0.93 (t, J1= J2=6.8Hz, 3H).
[0043] API-ES MS (m / z): 474.3 ([M+H] + ), 496.3 ([M+Na] + ).
Embodiment 3
[0045] Synthesis of 4-(p-hexyloxybenzoate)-N-n-hexyl-1,8-naphthalimide (N3)
[0046] Except that n-hexylamine was used instead of n-propylamine, other synthesis and purification methods were the same as in Example 1 to obtain the target product N3 with a yield of 46% and a melting point of 137.4-138.1°C.
[0047] 1 H-NMR (400MHz, CDCl 3 ), δH (ppm): 8.65 (d, J=8.0Hz, 2H), 8.31 (d, J=8.0Hz, 1H), 8.26 (d, J=8.8Hz, 2H), 7.76 (dd, J1=7.2 Hz, J2=8.0Hz, 1H), 7.66 (d, J=8.0Hz, 1H), 7.05 (d, J=8.8Hz, 2H), 4.18 (t, J1=J2=8.0Hz, 2H), 4.09 ( t, J1=J2=6.4Hz, 2H), 1.84 (m, 2H), 1.73 (m, 2H), 1.43 (m, 12H), 0.93 (t, J1=J2=7.2Hz, 3H), 0.88 (t , J1=J2=6.8Hz, 3H).
[0048] API-ES MS(m / z): 502.2([M+H] + ), 524.2 ([M+Na] + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com