Gel patch matrix and preparation method and application thereof
A gel patch and matrix technology, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. Less problems such as preventing skin residues, ensuring shapeability, and increasing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
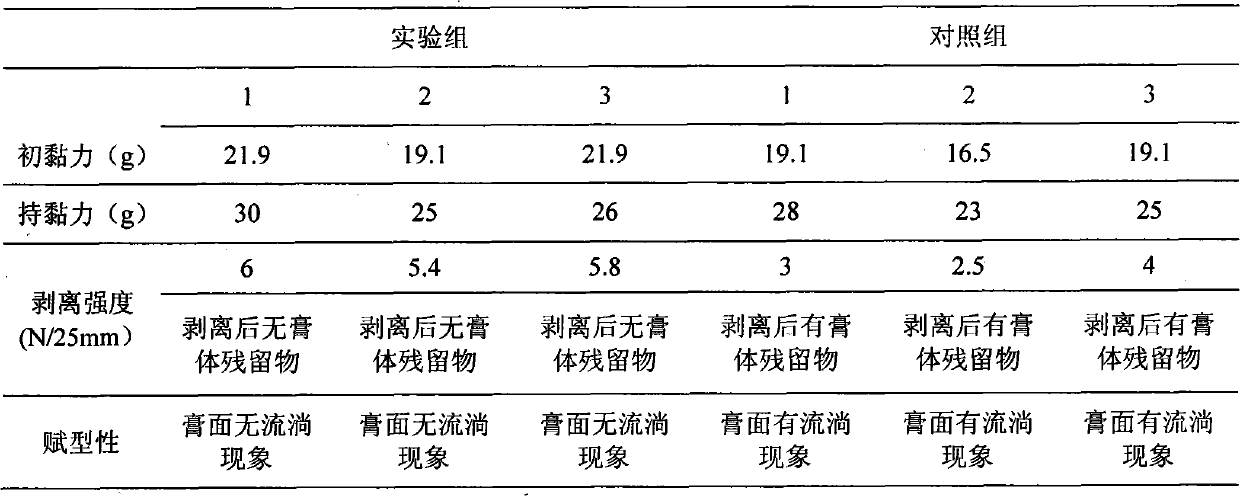
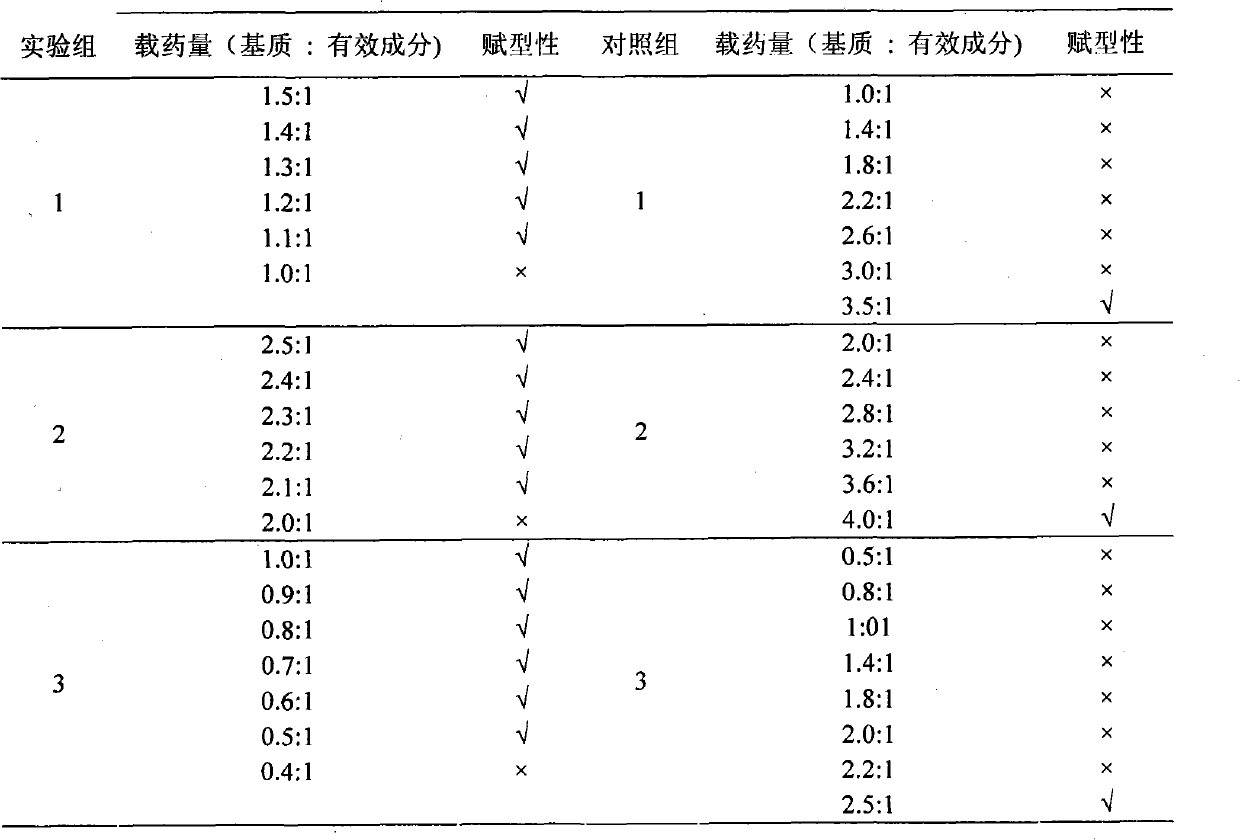
Examples
example 1
[0021] 1. Formula and proportion:
[0022] Sodium polyacrylate 6g, gelatin 5g, carbomer 980 2g, carbomer 981 2g, triethanolamine 5g, polyvinylpyrrolidone K-30 2.5g, glycerin 15g, aluminum glycinate 0.5g, water 150ml.
[0023] 2. Preparation method:
[0024] (1) Disperse sodium polyacrylate with glycerin, add 60ml of water and stir evenly, then add gelatin, heat to 90°C to dissolve, stir evenly to obtain phase I;
[0025] (2) Take Carbomer 980 and Carbomer 981 and add 60ml of water to swell for 12 hours, then add triethanolamine and stir well to obtain phase II;
[0026] (3) Take polyvinylpyrrolidone K-30 and add 30ml of water, heat to 90°C to dissolve, and obtain phase IV;
[0027] (4) Disperse aluminum glycinate in 10 mL of water to obtain phase IV;
[0028] (5) Add phase II and III into phase I, stir evenly, then add phase IV, stir evenly, add citric acid (50%) to adjust the pH to 6, and obtain.
example 2
[0030] 1. Formula and proportion:
[0031] Sodium polyacrylate 2g, gelatin 1g, carbomer 9804g, carbomer 9814g, triethanolamine 10g, polyvinylpyrrolidone K-305g, glycerin 5g, aluminum glycinate 0.1g, water 180mL.
[0032] 2. Preparation method:
[0033] (1) Disperse sodium polyacrylate with glycerin, add 20mL of water, stir until uniform, add gelatin, heat and melt at 90°C, and stir uniformly to obtain phase I;
[0034] (2) Carbomer 980 and 981 were swollen with 120 mL of water for 12 hours, then added triethanolamine and stirred to obtain phase II;
[0035] (3) Add 30 mL of water to polyvinylpyrrolidone K-30, heat and dissolve at 90°C to obtain phase IV;
[0036] (4) Disperse aluminum glycinate in 10 mL of water to obtain phase IV;
[0037] (5) Add phase II and III into phase I, stir evenly, then add phase IV, stir evenly, add citric acid (50%) to adjust the pH to 6.1, and obtain.
example 3
[0039] 1. Formula and proportion:
[0040] Sodium polyacrylate 10g, gelatin 8g, carbomer 980 0.5g, carbomer 981 0.5g, triethanolamine 1g, polyvinylpyrrolidone K-30 2g, glycerin 25g, aluminum glycinate 1g, water 145mL
[0041] 2. Preparation method:
[0042] (1) Disperse sodium polyacrylate with glycerin, add 100mL of water, stir until uniform, add gelatin, heat and melt at 90°C, and stir uniformly to obtain phase I;
[0043] (2) Add 15 mL of water to carbomer 980 and 981 to swell for 12 hours, then add triethanolamine and stir well to obtain phase II;
[0044] (3) Add 20 mL of water to polyvinylpyrrolidone K-30, heat and dissolve at 90°C to obtain phase IV;
[0045] (4) Disperse aluminum glycinate in 10 mL of water to obtain phase IV;
[0046] (5) Add phase II and III into phase I, stir evenly, then add phase IV, stir evenly, add citric acid (50%) to adjust the pH to 6.2, and obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com