Nickel complex and preparation and application thereof
A technology of metal complexes and nickel salts, which is applied in the field of nickel metal complexes and their preparation and application, and can solve the problems of difficult single catalysis of ethylene, increased process, and difficulty in improving the reactivity of ethylene, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
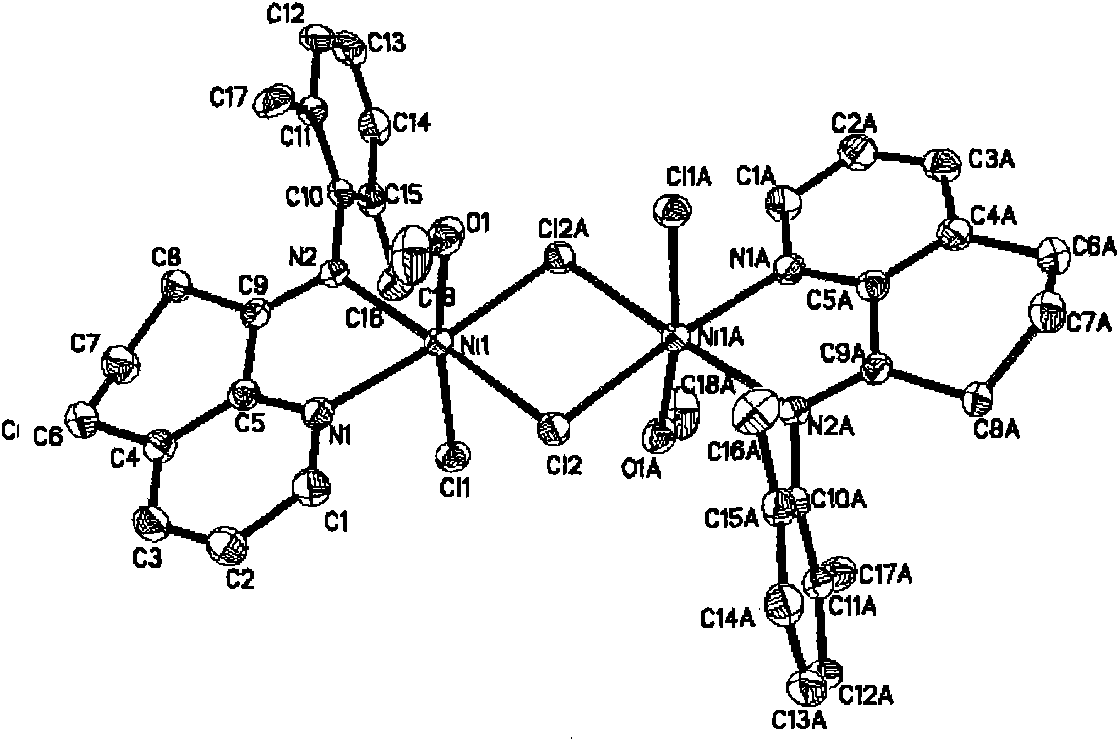
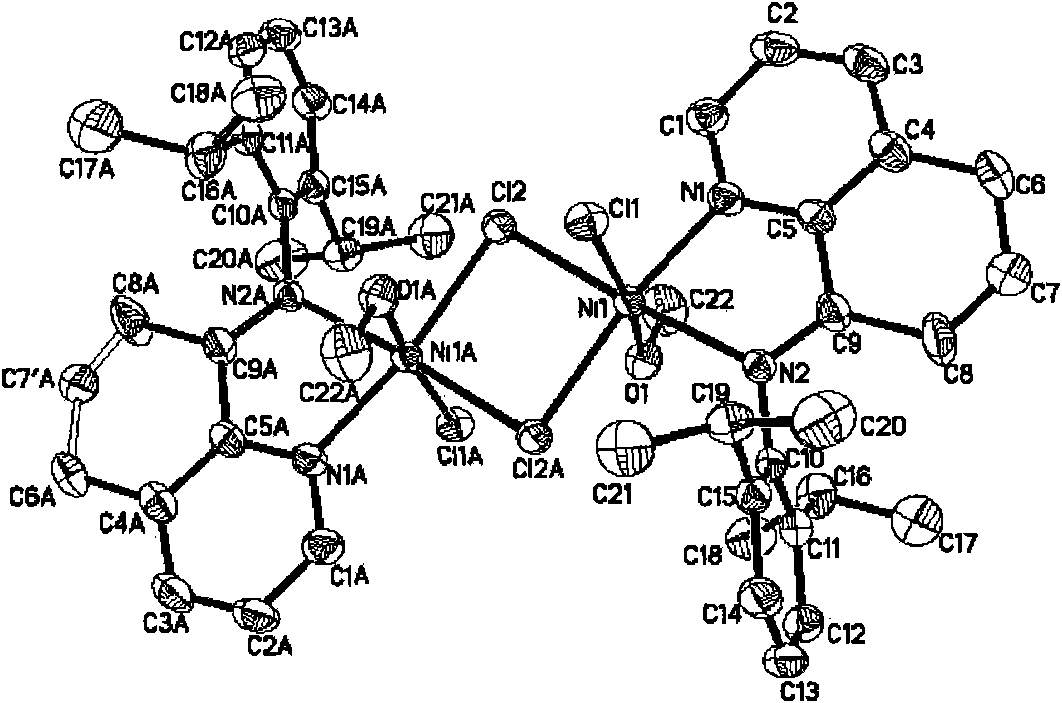
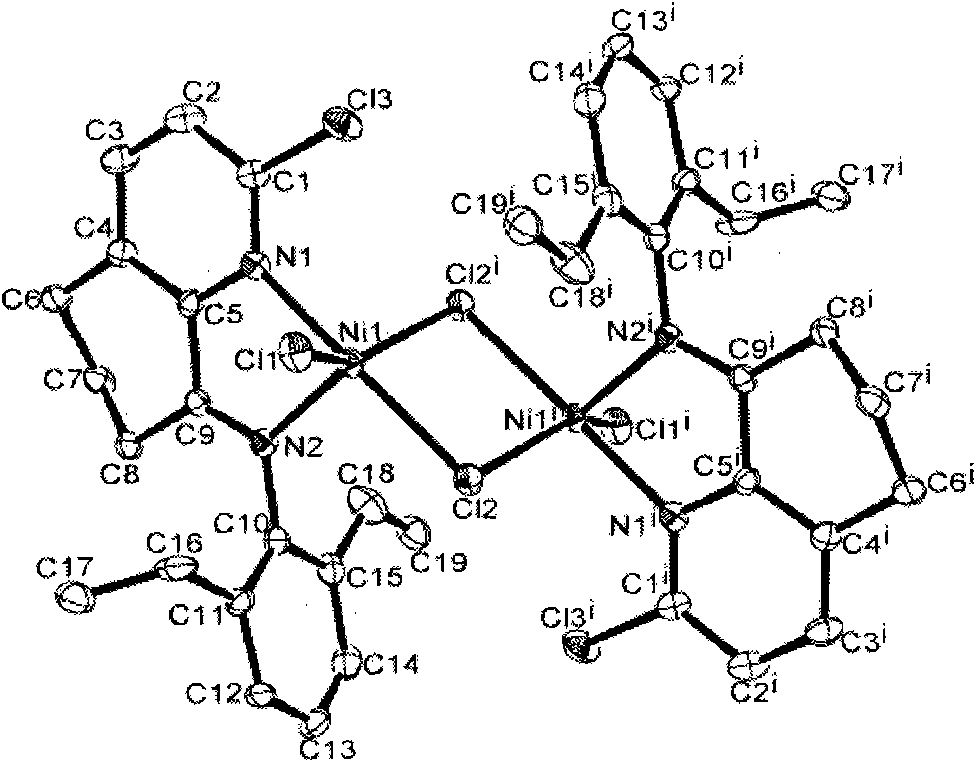
Image
Examples
Embodiment 1
[0042] 1. Preparation of ligand: Preparation of 5,6,7-trihydroquinoline-8-ketal 2,6-diisopropylaniline [L3]: 5,6,7-trihydroquinoline-8- Ketone (0.59 g, 5 mmol) was mixed with 2,6-diisopropylaniline (0.6 g, 5.1 mmol) in an equimolar ratio, and a catalytic amount of p-toluenesulfonic acid (86 mg, 0.5 mmol) was added , Reflux reaction in 100 ml of toluene for 3h, the reaction must be carried out under the protection of nitrogen. At the same time, a water separator can be added to increase the yield. After the reaction is completed, the alumina column chromatography is separated to obtain a yellow oily ligand with a yield of 66%. FT-IR (KBr, cm -1 ): 3047, 2960, 2866, 2829, 1929.3, 1864, 1640 (v C = N), 1571, 1476, 1426, 1329, 1254, 1194, 1104, 1050, 1016, 796, 771, 674. 1 H NMR (400MHz, CDCl 3 ): δ 8.73 (d, J = 4.1 Hz, 1H, Py H); 7.57 (d, J = 7.7Hz, 1H, Py H); 7.30 (t, J = 4.5Hz, 1H, Py H); 7.14 ( d, J = 7.5 Hz, 2H, Ar H); 7.07 (t, J = 6.7 Hz, 1H, Ar H); 2.95 (t, J = 6.0 Hz, ...
Embodiment 2
[0047] 1. The preparation of catalyst Ni3 is the same as that in Example 1.
[0048] 2. Pressurized ethylene polymerization: heat the polymerization kettle to 100°C, evacuate while hot and replace with nitrogen for 3 times. Under the condition of pre-replacing the nitrogen in the kettle with ethylene, the polymerization kettle was allowed to cool down slowly to the expected polymerization temperature (20° C.). Rinse still three times with toluene, then add 50mL toluene successively, 20 mL is dissolved with the toluene solution of 1.5 μ mol catalyst (Ni3), 0.35mL promoter (EASC, the toluene solution of 0.43mol / L), described catalyst center metal nickel and promoter The molar ratio of metal aluminum in the catalyst is about 1:200, and toluene remains (so that the total amount of toluene is 100 ml). The kettle was closed, and ethylene was passed through to maintain a constant pressure of ethylene (10 atm). After the polymerization reaction reaches the preset time (30min), the e...
Embodiment 3
[0050] 1. The preparation of catalyst Ni3 is the same as that in Example 1.
[0051] 2. Pressurized ethylene polymerization: heat the polymerization kettle to 100°C, evacuate while hot and replace with nitrogen for 3 times. Under the condition of pre-replacing the nitrogen in the kettle with ethylene, the polymerization kettle was allowed to cool down slowly to the expected polymerization temperature (20° C.). Rinse the kettle three times with toluene, then add 50mL toluene successively, 20mL is dissolved with the toluene solution of 1.5μmol catalyst (Ni3), 0.41mL co-catalyst (AlEt 3 , 0.74mol / L toluene solution), the molar ratio of the metal nickel in the catalyst center to the metal aluminum in the cocatalyst is about 1:200, and the remaining toluene (making the total amount of toluene 100 milliliters). The kettle was closed, and ethylene was passed through to maintain a constant pressure of ethylene (10 atm). After the polymerization reaction reaches the preset time (30mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com