Method for sustainably complementing metallic cations in plating solution
A technology of metal cations and cations, applied in the direction of cells, electrolytic processes, electrolytic components, etc., can solve the problems of wasting plating solution, environmental pollution of plating solution, waste of resources, etc., to reduce production costs, improve plating efficiency, and prolong service life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, electroplating metallic nickel;
[0020] A method for continuously replenishing metal cations (nickel ions) in the plating solution. During the electroplating process, the plating solution in the electroplating device is sent into the cation generator. When the cation generator works, nickel ions will be generated, and the The plating solution of nickel ions is sent back to the electroplating device, and circulates like this to supplement the consumed metallic nickel ions in the plating solution of the electroplating device.
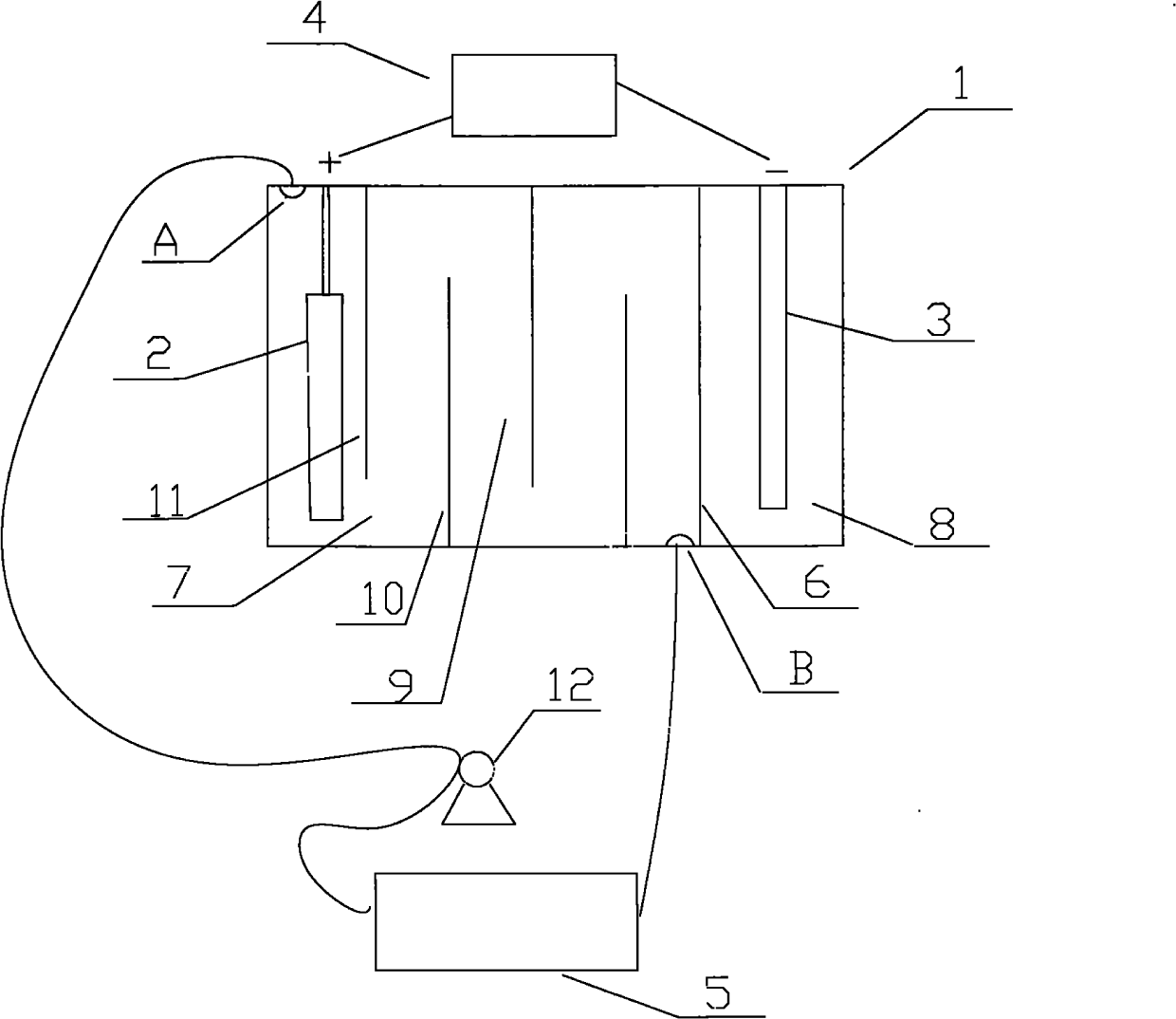
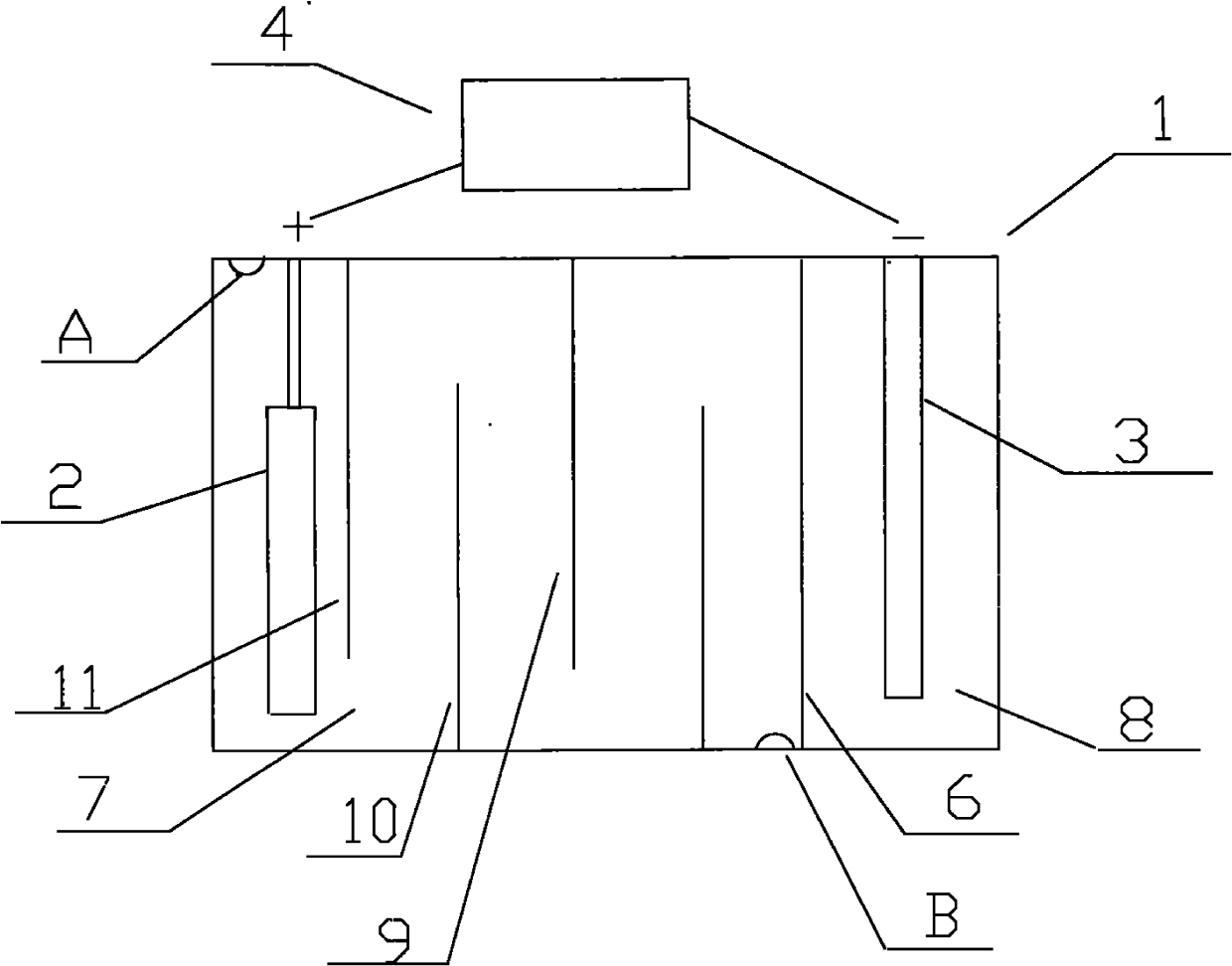
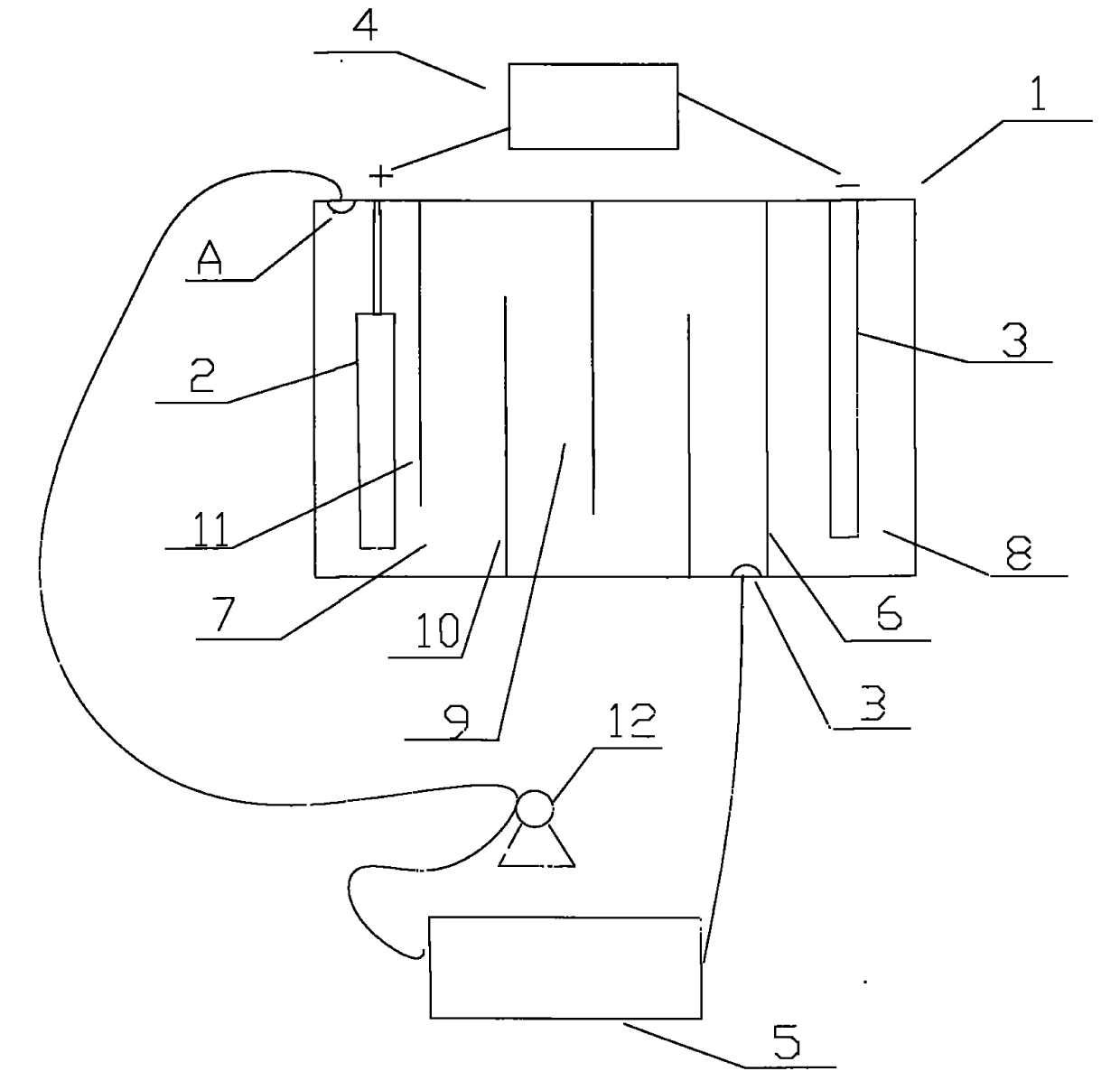
[0021] like figure 1 Shown, the cation generating device that the present invention adopts comprises electrolytic cell 1, and the anode 2 that is provided with in electrolytic cell 1 is a nickel post, and the negative electrode 3 that is provided with in electrolytic cell 1 is a graphite rod, also includes the connected anode 2 and negative electrode. 3 of the electrolysis power supply 4, the positive pole of the electrolysis power ...
Embodiment 2
[0024] Embodiment 2, electroplating metal copper;
[0025] A method for continuously replenishing metal cations (copper ions) in the plating solution. During the electroplating process, the plating solution in the electroplating device is sent into the cation generator. When the cation generator works, copper ions will be generated, and the The plating solution of copper ions is sent back to the electroplating device, and circulates like this to replenish the consumed metal copper ions in the plating solution of the electroplating device;
[0026] like figure 1 As shown, the cation generating device adopted in the present invention includes an electrolytic cell 1, in which an anode 2 is a copper column, and the others are the same as in Embodiment 1.
[0027] like figure 2 It further shows a method process of the present invention that can sustainably replenish metal cations (copper ions) in the plating solution. During electroplating, the cation generating device is used i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com