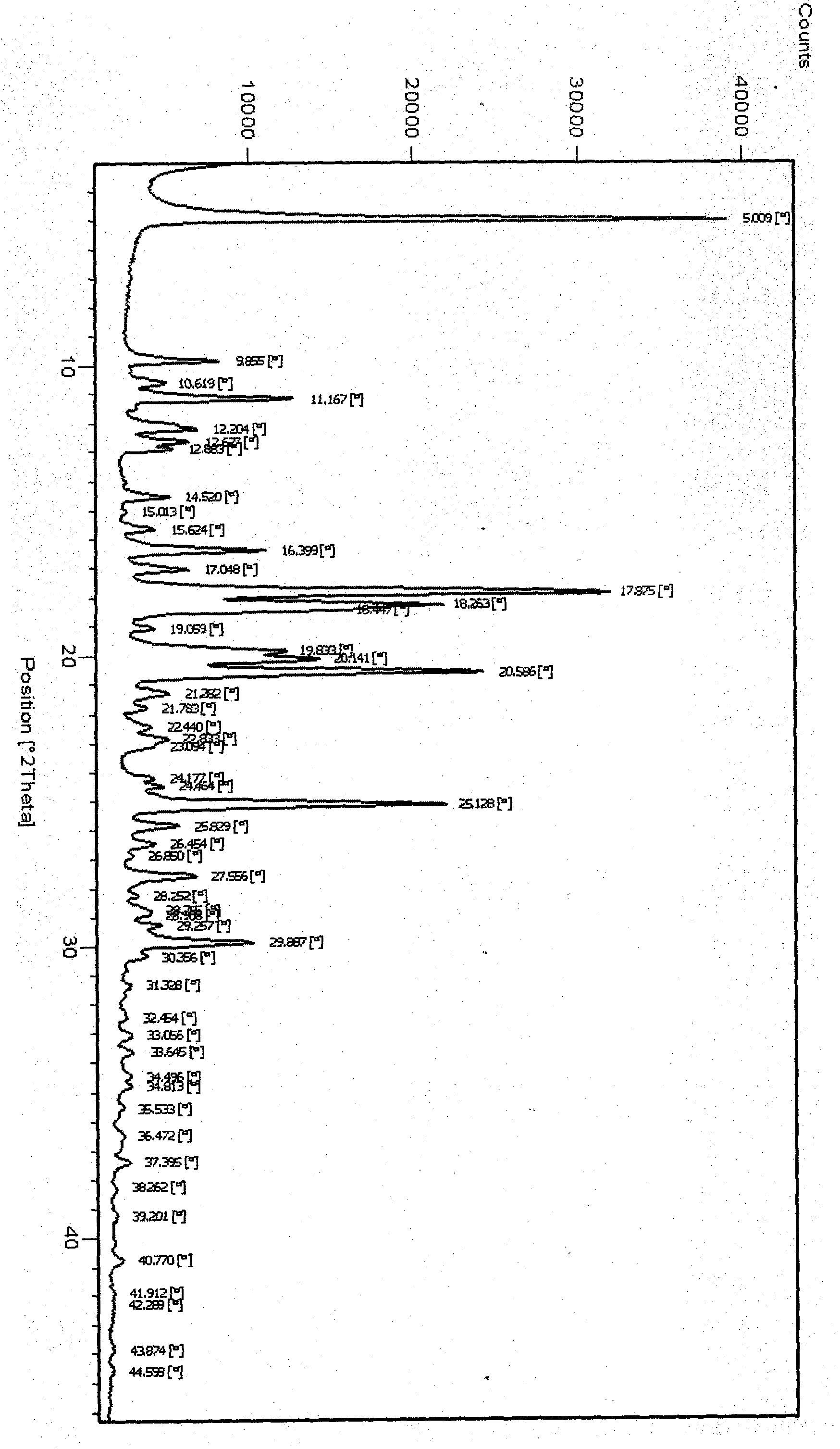
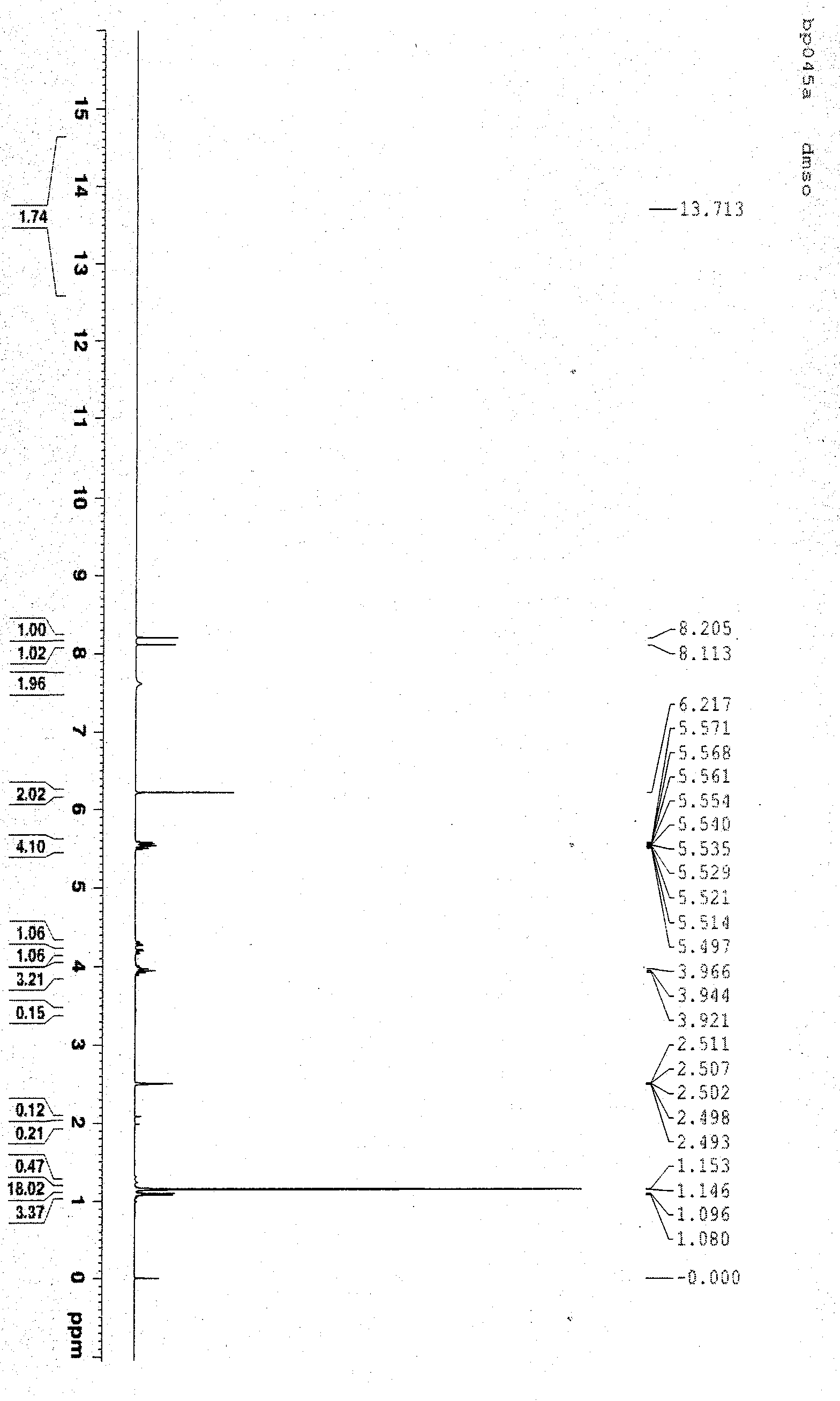
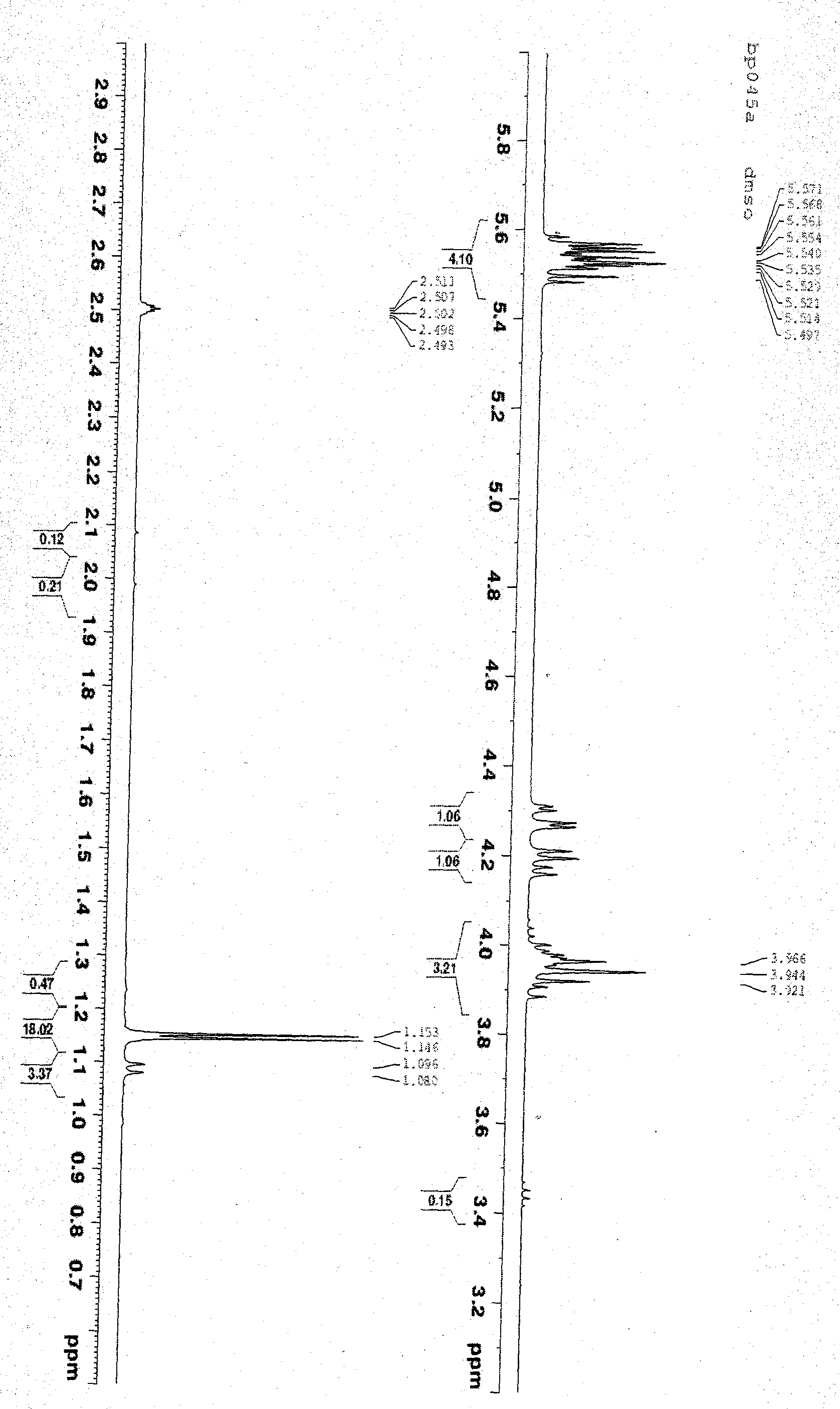
Novel crystal form of tenofovir ester maleate, preparation method and pharmaceutical application thereof
A technology of tenofovir dipivoxil and ester maleate, applied in the field of -phosphinoylmethoxy], can solve problems such as restricting industrialized large-scale production, stability problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis and crystallization purification of (R)-9-[2-bis(pivaloyloxymethyl)phosphonomethoxypropyl]adenine (tenofovir dipivoxil)
[0028] Mix PMPA (5g), chloromethyl tert-butyl ester (13.79mL), triethylamine (7.64mL) and N-methylpyrrolidone (19g) under nitrogen protection and heat to 60±3°C (no more than 65 ℃). After 2 hours, TLC (dichloromethane / methanol=15 / 1), showed that the starting material had disappeared. Lower the temperature of the reactant to room temperature (25±2°C), add ethyl acetate (50mL) and water (30mL) and stir for 30 minutes, separate the liquids, continue to back-extract the aqueous phase once with ethyl acetate (50mL), and combine ethyl acetate The ester phase was washed once with saturated aqueous sodium chloride (20 mL), and dried over anhydrous sodium sulfate to remove water. At a temperature not higher than 50°C, the organic solvent was evaporated in vacuo to obtain 10 g of a viscous yellow oil, which was purified by column to obtai...
Embodiment 2
[0091] White foamy solid (3 g), maleic acid (0.34 g), and ethanol (20 mL) were heated to 50° C. and stirred for 1 hour, then filtered while hot. The mother liquor was evaporated in vacuo to remove the organic solvent at a temperature of 30° C. to obtain a slightly yellow oil (3.3 g). Dissolve the oil with hot ethyl acetate (200 mL, temperature about 50°C), mix and cool to room temperature, add seed crystals, place at 10°C, filter after 12 hours, wash with a small amount of ethyl acetate to obtain a white solid.
Embodiment 3
[0093] White foamy solid (3 g), maleic acid (0.39 g), and ethanol (20 mL) were heated to 60°C and stirred for 1 hour, then filtered while hot. The mother liquor was evaporated in vacuo to remove the organic solvent at a temperature of 30° C. to obtain a slightly yellow oil (3.3 g). Dissolve the oil with hot ethyl acetate (200 mL, temperature about 40°C), mix and cool to room temperature, add seed crystals, place at 10°C, filter after 18 hours, wash with a small amount of ethyl acetate to obtain a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com