Method for preparing hydrogen chloride and ammonia by utilizing ammonium chloride
A technology of ammonium chloride and hydrogen chloride, applied in the direction of chlorine/hydrogen chloride, preparation with chloride, preparation/separation of ammonia, etc., can solve problems such as side reactions, low purity, low reaction rate of acid release, etc., and achieve increased reaction speed , high initial purity, and the effect of reducing the amount of sublimation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
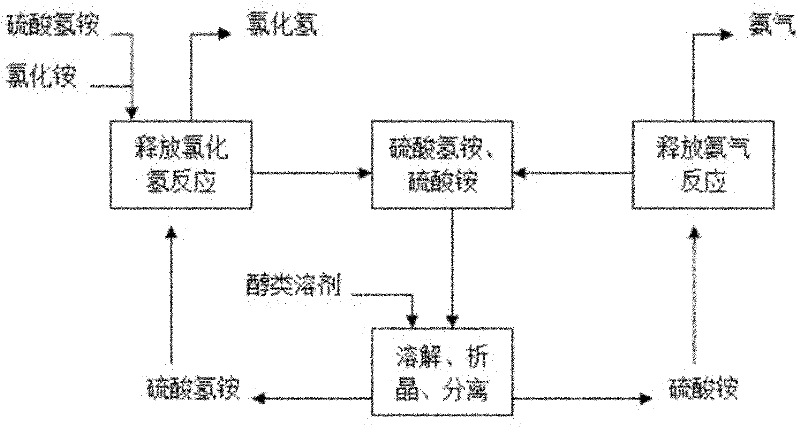
[0034] Such as figure 1 Shown, a kind of ammonium chloride produces the method for hydrogen chloride and ammonia, comprises the following steps:
[0035] 1. Acid release reaction process
[0036] During the acid release reaction, first add the intermediate separation inorganic acidic solvent ammonium bisulfate to the reaction kettle, after heating up to 180°C, add the dried and sieved solid ammonium chloride with a particle size of 40 mesh, hydrogen sulfate The molar ratio of ammonium to ammonium chloride is 1:1. At this time, a large amount of hydrogen chloride gas is produced. After 2 hours of constant temperature reaction, the conversion rate of ammonium chloride reaches 90.5%. The hydrogen chloride gas produced has a purity of 91.8% without treatment and can be used after treatment. In the production or preparation of high-purity hydrochloric acid in PVC, the remaining ammonium bisulfate and ammonium sulfate are crystallized and separated;
[0037] 2. Intermediate separa...
Embodiment 2
[0042] A method for ammonium chloride to produce hydrogen chloride and ammonia, comprising the following steps:
[0043] 1. Acid release reaction process
[0044]During the acid release reaction, first add the intermediate separation inorganic acidic solvent ammonium bisulfate to the reaction kettle, after heating up to 250°C, add the dried and sieved solid ammonium chloride with a particle size of 100 mesh, hydrogen sulfate The molar ratio of ammonium to ammonium chloride is 5:1. At this time, a large amount of hydrogen chloride gas is produced. After 2 hours of constant temperature reaction, the conversion rate of ammonium chloride reaches 91.5%. The hydrogen chloride gas produced has a purity of 93.2% without treatment and can be used after treatment In the production or preparation of high-purity hydrochloric acid in PVC, the remaining ammonium bisulfate and ammonium sulfate are crystallized and separated;
[0045] 2. Intermediate separation process
[0046] The molten m...
Embodiment 3
[0050] A method for ammonium chloride to produce hydrogen chloride and ammonia, comprising the following steps:
[0051] 1. Acid release reaction process
[0052] During the acid release reaction, first add the intermediate separation inorganic acidic solvent ammonium bisulfate to the reaction kettle, after heating up to 220°C, add the dried and sieved solid ammonium chloride with a particle size of 60 mesh, hydrogen sulfate The molar ratio of ammonium to ammonium chloride is 3:1. At this time, a large amount of hydrogen chloride gas is produced. After 2 hours of constant temperature reaction, the conversion rate of ammonium chloride reaches 90.8%. The hydrogen chloride gas produced has a purity of 92.6% without treatment and can be used after treatment In the production or preparation of high-purity hydrochloric acid in PVC, the remaining ammonium bisulfate and ammonium sulfate are crystallized and separated;
[0053] 2. Intermediate separation process
[0054] The molten m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com