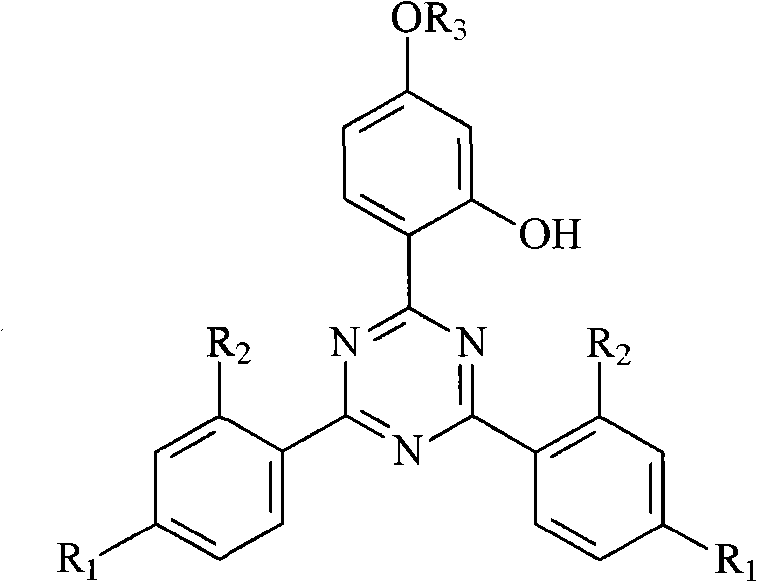
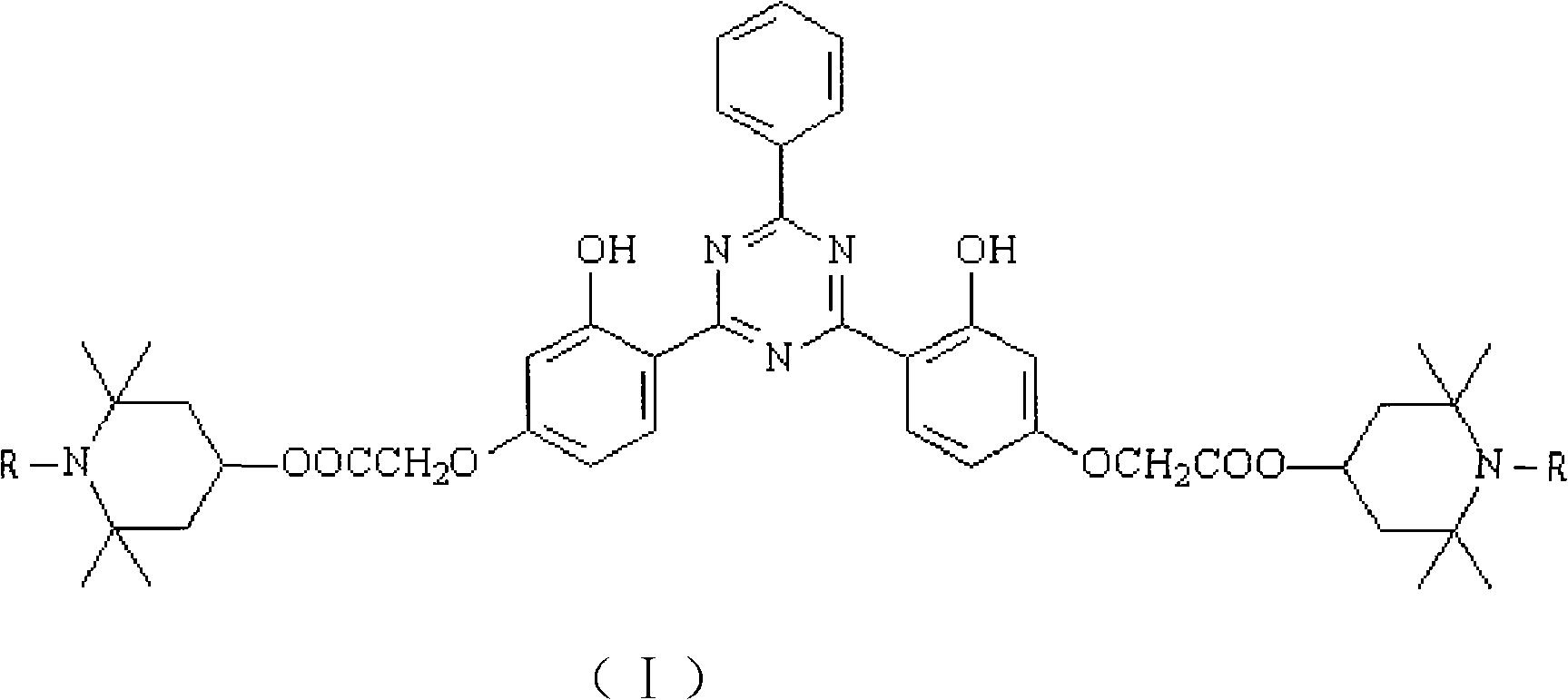
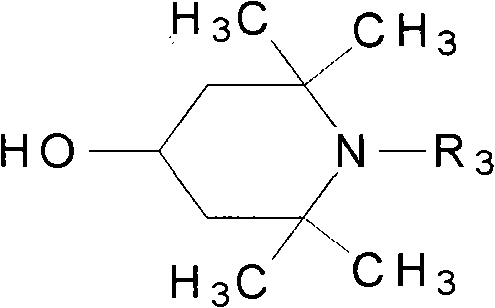
Triazine light stabilizer containing hindered amine groups
A light stabilizer, hindered amine technology, applied in the field of light stabilizers, can solve the problem of no obvious improvement in absorption intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]Add 10.0 g (0.0183 mol) 2-phenyl-4,6-di(2-hydroxy-4-ethoxycarbonylmethoxyphenyl)- 1,3,5-triazine, 9g (0.0573mol) 2,2,6,6-tetramethylpiperidinol, 0.3g dibutyltin oxide and 50mL xylene were reacted at 140°C for 8h. After the reaction was completed, the temperature was lowered, filtered, dried, and recrystallized from N,N-dimethylformamide to obtain a yellow solid, namely 2-phenyl-4,6-bis[2-hydroxyl-4-(2,2,6 , 6-tetramethylpiperidine-4-oxyl)carbonylmethoxyphenyl]-1,3,5-triazine (II) 9.1g, yield 64.7%, high performance liquid chromatography (HPLC) purity 98.7 %, melting point 114.0-115.3°C, MS molecular ion peak 767.5.
[0029] Elemental Analysis Results:
[0030]
Embodiment 2
[0032] Add 10.0 g (0.0183 mol) 2-phenyl-4,6-di(2-hydroxy-4-ethoxycarbonylmethoxyphenyl)- 1,3,5-triazine, 10g (0.0585mol) 1,2,2,6,6-pentamethylpiperidinol, 0.3g dibutyltin oxide and 50mL xylene were reacted at 140°C for 8h. After the reaction was completed, the temperature was lowered, filtered, dried, and then recrystallized with N,N-dimethylformamide to obtain a yellow solid, that is, 2-phenyl-4,6-bis[2-hydroxyl-4-(1,2,2 , 6,6-tetramethylpiperidine-4-oxyl)carbonylmethoxyphenyl]-1,3,5-triazine (III) 8.7g, yield 59.6%, high performance liquid chromatography (HPLC) The purity is 98.1%, the melting point is 202.3-203.2°C, and the MS molecular ion peak is 795.5.
[0033] Elemental Analysis Results:
[0034]
Embodiment 3
[0036] Add 10.0 g (0.0183 mol) 2-phenyl-4,6-di(2-hydroxy-4-ethoxycarbonylmethoxyphenyl)- 1,3,5-Triazine, 11g (0.0457mol) N-hexyl-2,2,6,6-pentamethylpiperidinol, 0.2g tetrabutyl titanate and 30mL chlorobenzene were reacted at 120°C for 8h. After the reaction was completed, the temperature was lowered, filtered, dried, and recrystallized from N,N-dimethylformamide to obtain a yellow solid, namely 2-phenyl-4,6-bis[2-hydroxyl-4-(1-hexyl-2 , 2,6,6-tetramethylpiperidine-4-oxyl)carbonylmethoxyphenyl]-1,3,5-triazine (IV) 9.1g, yield 53.1%, HPLC ( HPLC) purity 98.2%, melting point 195.3-196.2°C, MS molecular ion peak 935.5.
[0037] Elemental Analysis Results:
[0038]
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com