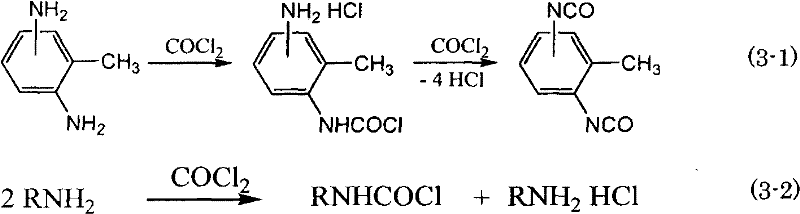
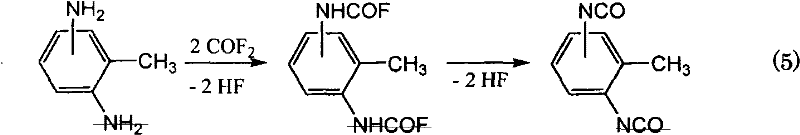
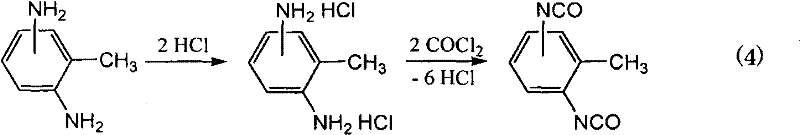Method for producing isocyanate compound
A technology of isocyanate and naphthalene diisocyanate, applied in the novel preparation field, can solve the problem of no carbonyl fluoride technology research, etc., and achieve the effects of low toxicity and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] The synthesis of embodiment 1 toluene di(carbamoyl fluoride)
[0100] 1 g of 2,4-toluenediamine and COF 2 5g in chlorobenzene, stirring at room temperature, the resulting product precipitated out. This was filtered to give 1.6 g of solid product. Determination of the resulting product 1 H-, 19 F-NMR, and IR spectrum confirmed that 2,4-toluene di(carbamoyl fluoride) (2,4-(FCONH) 2 C 6 h 3 CH 3 ).
[0101] 1 H-NMR (DMF-d 7 , TMS): δ2.3 (s, 3H), 7.2-7.8 (m, 3H), 10.0 (br, 1H), 10.7 (br, 1H)
[0102] 19 F-NMR (DMF-d 7 , CFCl3): φ6.9 (s, 1F), 10.3 (s, 1F)
[0103] IR: 3289, 1808, 1797, 1770, 1550cm-1
Embodiment 2
[0104] The synthesis of embodiment 2 diphenylmethane two (carbamoyl fluorides)
[0105] 1 g of 4,4'-diphenylmethanediamine and COF 2 3.5 g was dissolved in chlorobenzene, stirred at room temperature, and the resulting product precipitated out. It was filtered to obtain 1.0 g of solid product. Determination of the resulting product 1 H-, 19 F-NMR, and IR spectra confirmed the formation of 4,4'-diphenylmethane bis(carbamoyl fluoride) (4,4'-FCONHC 6 h 4 CH 2 C 6 h 4 NHCFO).
[0106] 1 H-NMR (CDCl 3 , TMS): δ3.9 (s, 2H), 6.7 (br, 2H), 7.2 (m, 4H), 7.3 (m, 4H)
[0107] 19 F-NMR (CDCl 3 , CFCl3): φ6.8 (s, 1F)
[0108] IR: 3325, 1815, 1777, 1527cm-1
Embodiment 3
[0109] Embodiment 3 adopts one-step method to synthesize TDI
[0110] 1 g of 2,4-toluenediamine and COF 2 5 g was reacted in chlorobenzene with stirring at 150° C., and then the resulting product was analyzed using a gas chromatograph. Compared with commercially available samples, it was confirmed that 2,4-toluene diisocyanate (2,4-(NCO) 2 C 6 h 3 CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com