Catalytic synthesis method of unsaturated alcohol
A synthesis method and unsaturated technology, which are applied in the field of catalytic synthesis of unsaturated alcohols, can solve problems such as lack of industrialization conditions, and achieve the effects of high selectivity, simple operation and high product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
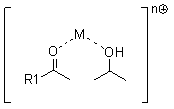
Method used
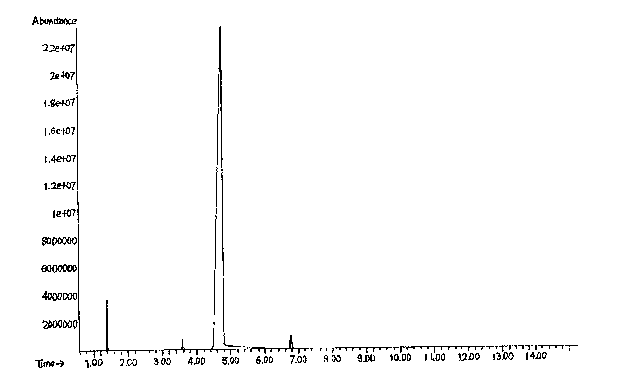
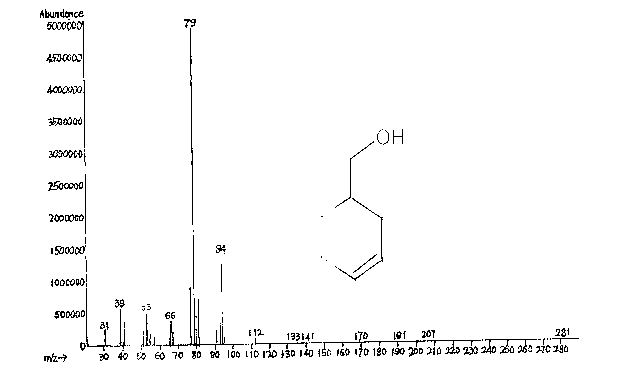
Image
Examples
Embodiment 1
[0023] In this embodiment, aluminum isopropoxide is used as a catalyst, LiBr is used as an auxiliary agent, and 3-cyclohexene-1-carbaldehyde is reduced to 3-cyclohexene-1-methanol in a solvent of isopropanol under a nitrogen atmosphere.
[0024] Add 4.8 g of aluminum isopropoxide, 1.2 g of LiBr, and 300 g of isopropanol to a 1 L flask. The temperature was raised to 80°C under nitrogen atmosphere. A solution of 60 g of 3-cyclohexene-1-carbaldehyde (0.55 mol) in isopropanol was added dropwise. During the dropwise addition process, the temperature of the system will decrease due to the generation of acetone with low boiling point. Evaporate the generated acetone while adding dropwise, carefully control the top temperature below 85 degrees, and use a thorn-type distillation column to increase the separation of acetone and isopropanol and control the amount of solvent evaporated. Gas chromatography was followed until the chromatographic content of 3-cyclohexene-1-carbaldehyde was...
Embodiment 2
[0027] In this example, the aluminum isopropoxide derivative derived from dihydric phenol is used as catalyst, and LiBr is used as auxiliary agent. Under nitrogen atmosphere, 3-cyclohexene-1-carbaldehyde is reduced to 3-cyclohexyl in solvent isopropanol En-1-methanol.
[0028] Add 3.0 g of dihydric phenol-derived aluminum isopropoxide derivative, 1.2 g of LiBr, and 300 g of isopropanol in a 1 L flask. The temperature was raised to 60°C under nitrogen atmosphere. A solution of 60 g of 3-cyclohexene-1-carbaldehyde (0.55 mol) in isopropanol was added dropwise. During the dropwise addition process, the temperature of the system will decrease due to the generation of acetone with low boiling point. Evaporate the generated acetone while adding dropwise, carefully control the top temperature below 85 degrees, and use a thorn-type distillation column to increase the separation of acetone and isopropanol and control the amount of solvent evaporated. Gas chromatography was followed u...
Embodiment 3
[0030] In this embodiment, aluminum sec-butoxide is used as a catalyst, and MgBr 2 As an auxiliary agent, reduce cinnamaldehyde to cinnamyl alcohol in the solvent 2-butanol under nitrogen atmosphere.
[0031] In a 1L flask was added 6.6 g of aluminum sec-butoxide, 2.6 g of MgBr 2 , 300 g of 2-butanol. The temperature was raised to 110°C under a nitrogen atmosphere. A solution of 66 g of cinnamaldehyde (0.5 mol) was added dropwise. During the dropwise addition process, the temperature of the system will decrease due to the generation of 2-butanone with low boiling point. Evaporate the generated 2-butanone while adding dropwise, carefully control the top temperature below 100 degrees, and use a thorn-type distillation column to increase the separation of 2-butanone and 2-butanol, and control the amount of solvent evaporated . Gas chromatography followed up until the chromatographic content of cinnamaldehyde was lower than 0.5%, and the reaction time was about 1-2 hours. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com