Preparation method of 11 alpha,17 alpha-dyhydroxyl-androst-4-ene-3,20-dione
A technology of dihydroxy and androster, which is applied in the field of 11α, can solve problems such as the inability to realize industrial production, and achieve the effect of increasing the conversion rate and increasing the concentration of substrate feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
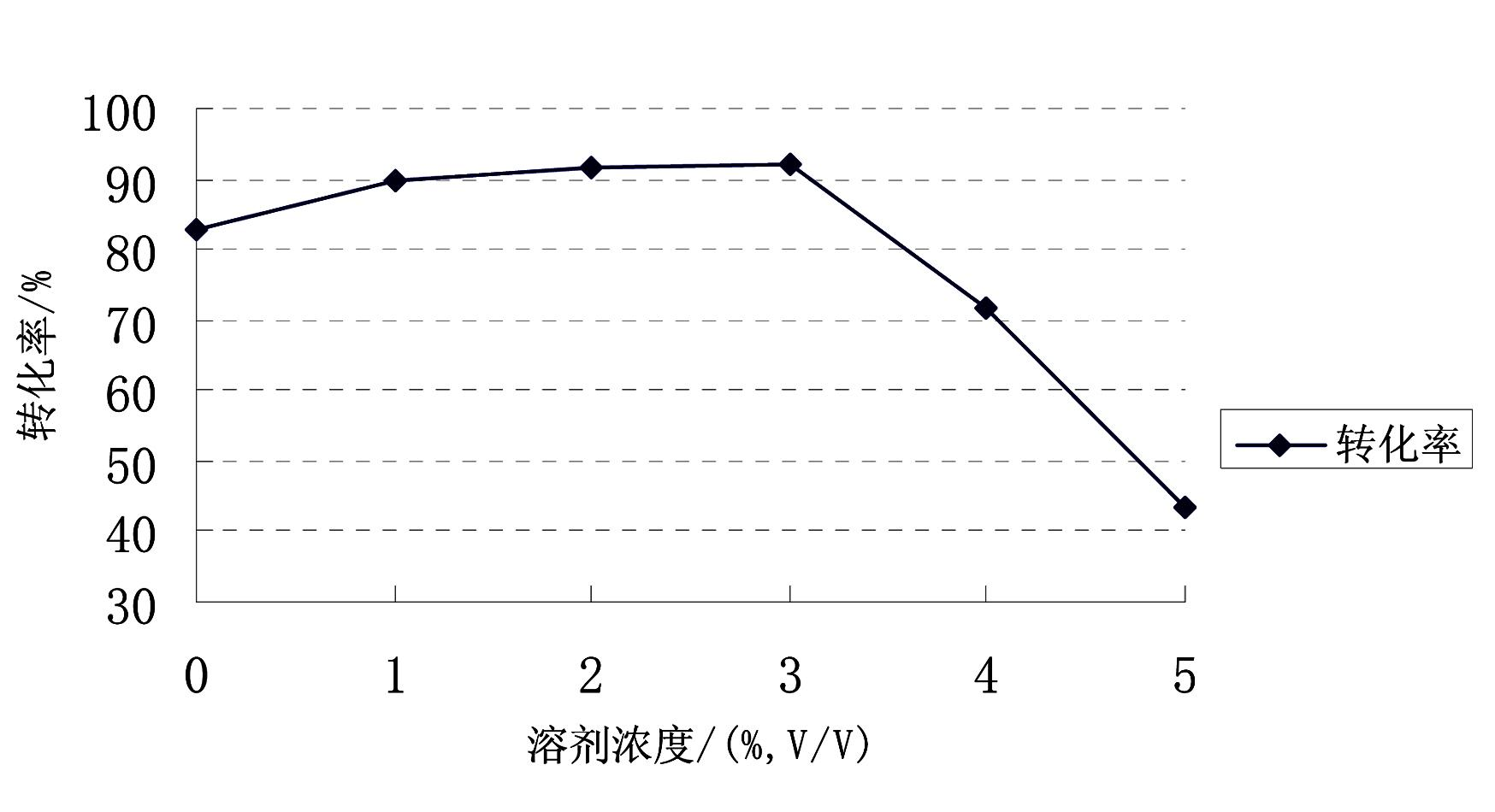
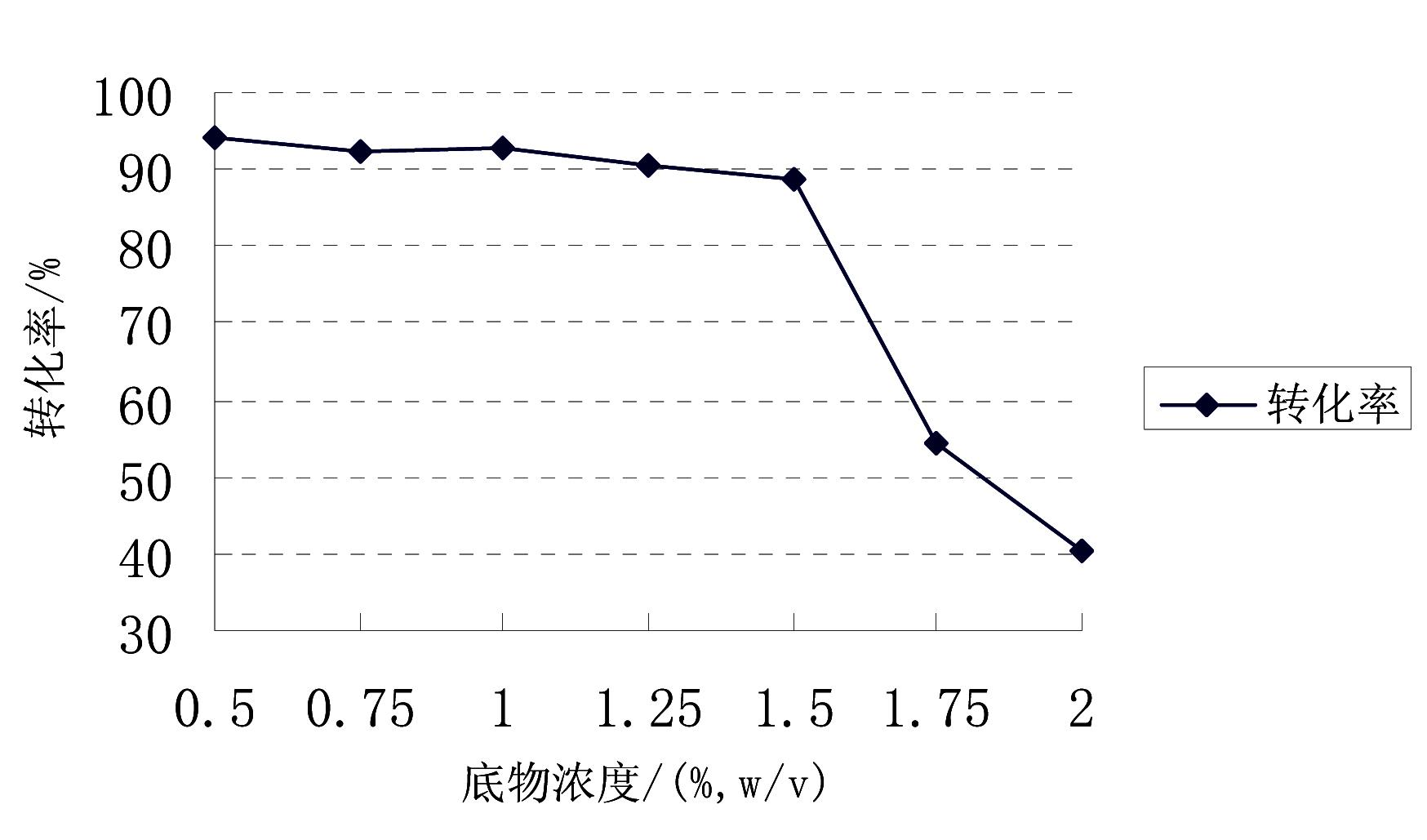
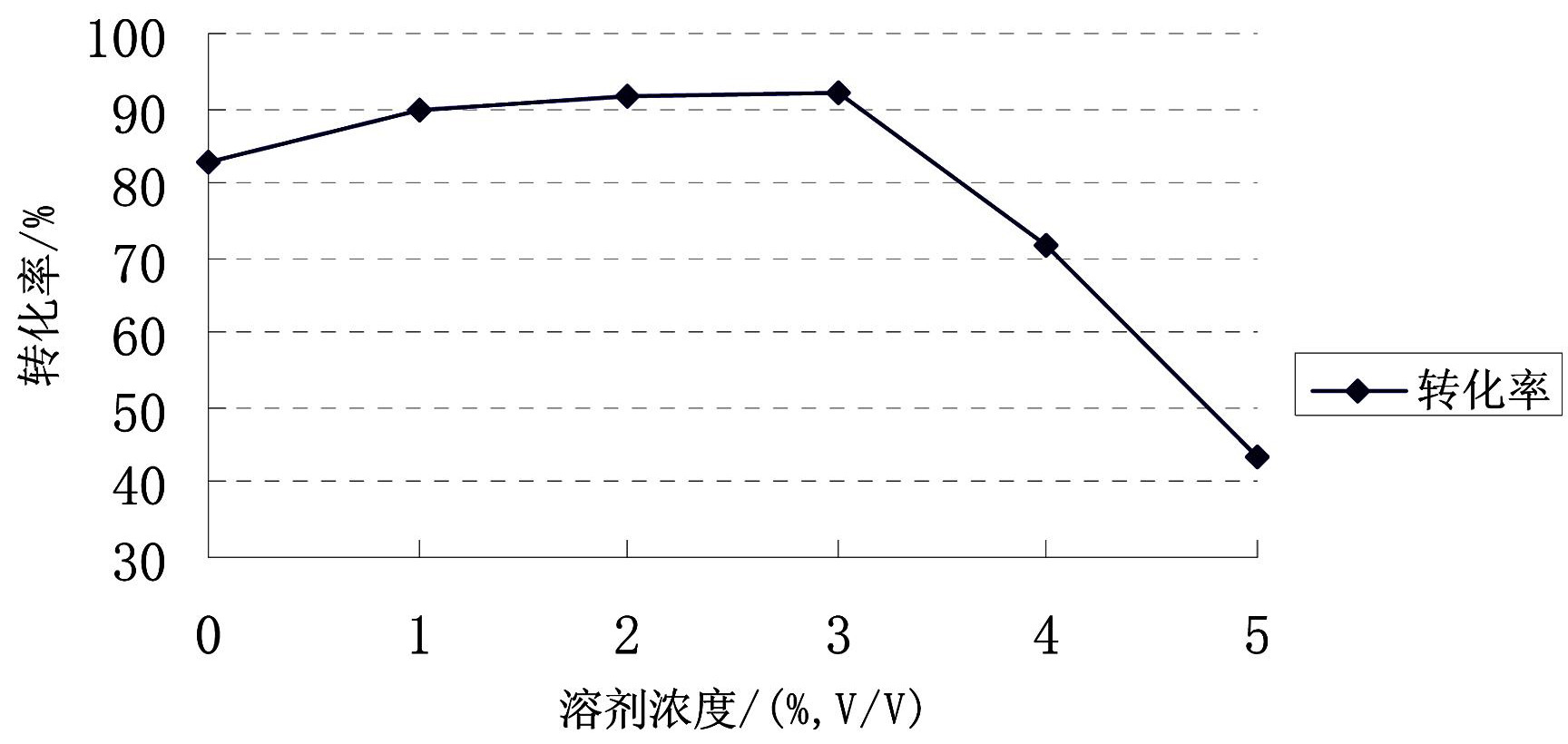
Image
Examples
Embodiment 1
[0062] 1000ml of medium was contained in a 2-liter flask, and the medium included 2% (weight percent) glucose, 2.2% (weight percent) corn steep liquor, 0.11% (weight percent) (NH 4 ) 2 SO 4 ; Sterilize the medium at 120°C for 30 minutes in an autoclave; cool down, and then inoculate the slant culture of Ochraus strains in the medium; inoculate the medium inoculated with the strain at 30±1°C in a shaker Cultivate for 24 hours at 165rpm;
[0063] After such precultivation, inoculate again in the 20 liters fermentor that is covered with 19 liters and the aseptic medium of precultivation final composition identical as above-mentioned; Add 1.0ml foam enemy additionally to reduce foaming;
[0064] After a 12-hour growth period at an overpressure of 0.4-0.6 bar and a temperature of 30±1°C, aerate at 4 liters / min and stir at 165 rpm;
[0065] Add 150g of 17α-hydroxy-androst-4-ene-3,20-dione (intermediate A) to 600ml of ethanol to dissolve, then put it into the culture medium; conti...
Embodiment 2
[0069] 1000ml of culture medium was housed in a 2-liter flask, and the culture medium included 2% (weight percent) glucose, 2% (weight percent) corn steep liquor, 0.10% (weight percent) (NH 4 ) 2 SO 4 ; Sterilize the medium at 120°C for 30 minutes in an autoclave; cool down, then inoculate the slant culture of Metarhizium anisopliae strains in the medium; inoculate the medium inoculated with the strain at 28±1°C in a shaker Cultivate at 165rpm for 24 hours;
[0070] After such precultivation, inoculate again in the 20 liters fermentor that is covered with 19 liters and the aseptic medium of precultivation final composition identical as above-mentioned; Add 1.0ml foam enemy additionally to reduce foaming;
[0071] After a 12-hour growth period at an overpressure of 0.4-0.6 bar and a temperature of 28±1°C, aerate at 4 liters / min and stir at 165 rpm;
[0072] Add 200g of 17α-hydroxy-androst-4-ene-3,20-dione (intermediate A) to 600ml dimethylformamide (DMF) to dissolve, then pu...
Embodiment 3
[0076] 1000ml of medium was housed in a 2-liter flask, and the medium included 3% (weight percent) glucose, 3% (weight percent) corn steep liquor, 0.13% (weight percent) (NH 4 ) 2 SO 4 ; Sterilize the medium at 120°C for 30 minutes in an autoclave; cool down, then inoculate the slant culture of Rhizopus niger strain in the medium; inoculate the medium inoculated with the strain at 26±1°C in a shaker Cultivate at 165rpm for 24 hours;
[0077] After such precultivation, inoculate again in the 20 liters fermentor that is covered with 19 liters and the aseptic medium of precultivation final composition identical as above-mentioned; Add 1.0ml foam enemy additionally to reduce foaming;
[0078] After a 12-hour growth period at an overpressure of 0.4-0.6 bar and a temperature of 26±1°C, aerate at 4 liters / min and stir at 165 rpm;
[0079] Add 100g of 17α-hydroxy-androst-4-ene-3,20-dione (intermediate A) into 600ml of methanol to dissolve, and then put it into the culture medium; c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com