Impurity analysis and preparation method for clindamycin phosphate
A technology for clindamycin phosphate and impurity analysis, applied in the fields of analytical chemistry and pharmaceutical analytical chemistry, which can solve the problems of lowering the limit of impurities, seldom considering the adverse effects of impurities on drug safety, and insufficiently comprehensive and accurate impurity control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0085] In order to better understand the technical solution of the present invention, the technical solution of the present invention will be further described below in conjunction with specific embodiments of the present invention, but it does not limit the present invention.
[0086] The raw materials of clindamycin phosphate used in this embodiment are all raw materials of the same name provided by Zhejiang Tiantai Pharmaceutical Co., Ltd.
[0087] Determination of Clindamycin Phosphate API by LC-MS
[0088] In order to study the impurities of Clindamycin Phosphate API, the main impurities of the API were studied by liquid chromatography-mass spectrometry, the purpose of which was to determine the molecular weight and chromatographic retention behavior of the impurities.
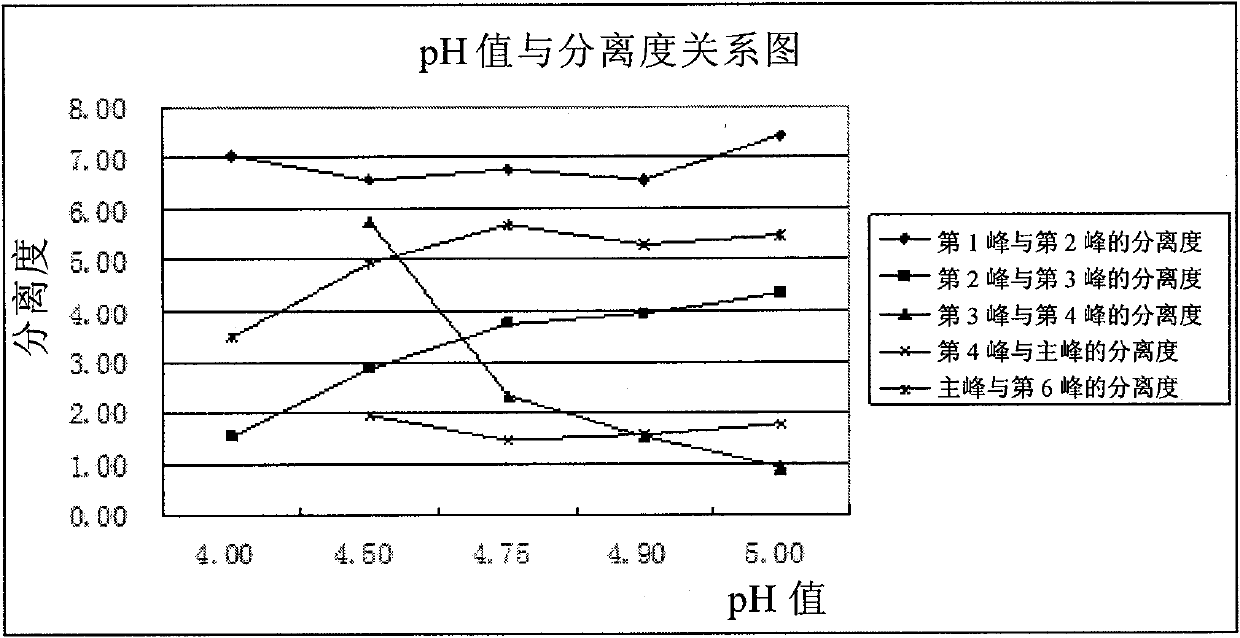
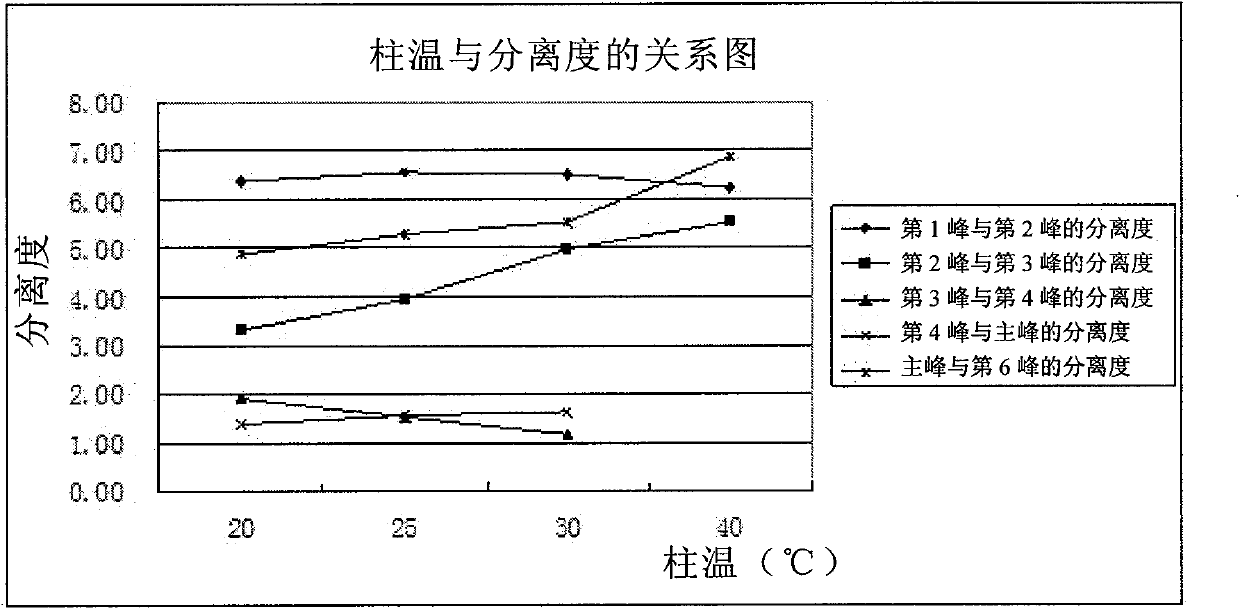
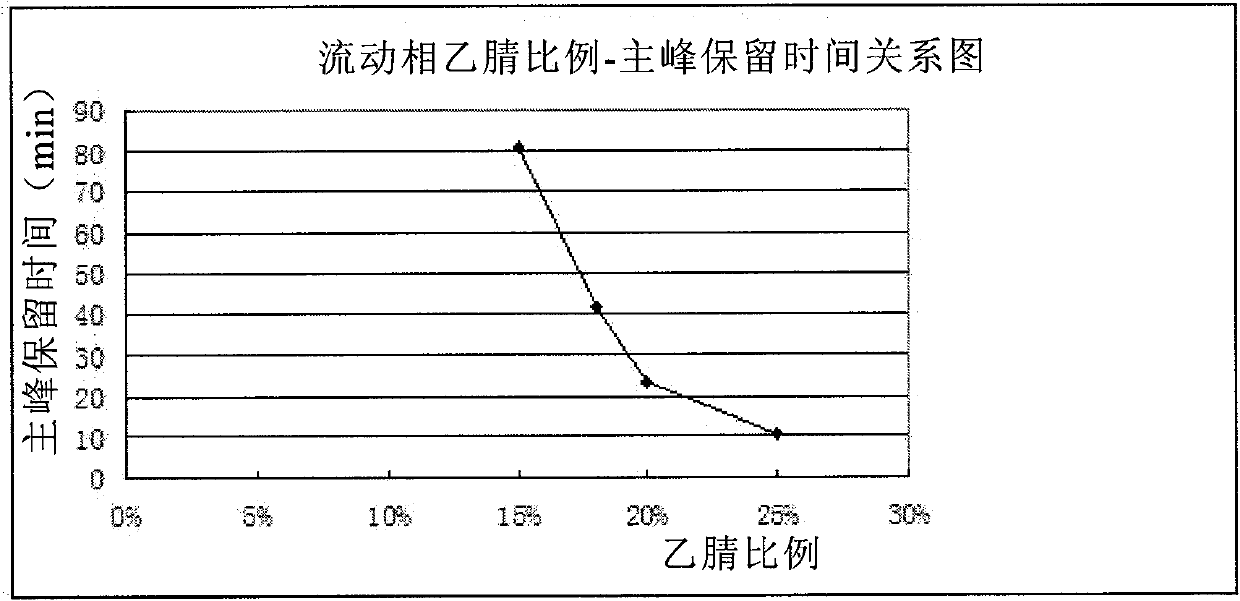
[0089] There are acid groups and basic groups in the molecular structure of clindamycin phosphate, which belongs to amphoteric substances. In the HPLC detection of various national pharmacopoeias, ion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com