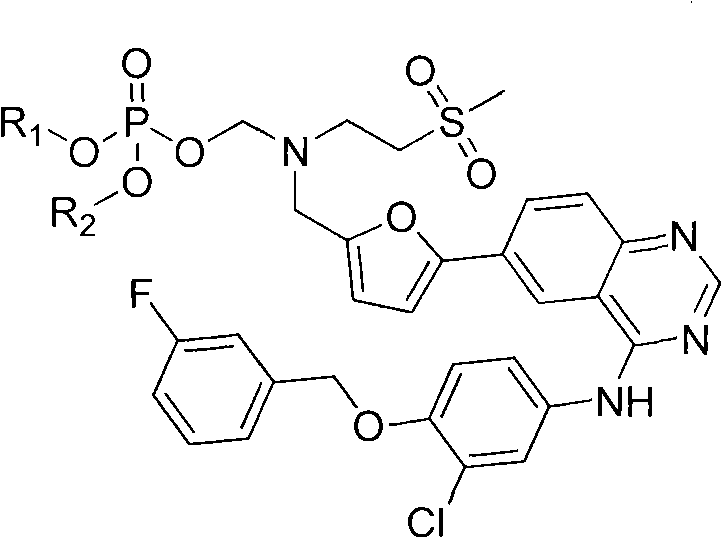
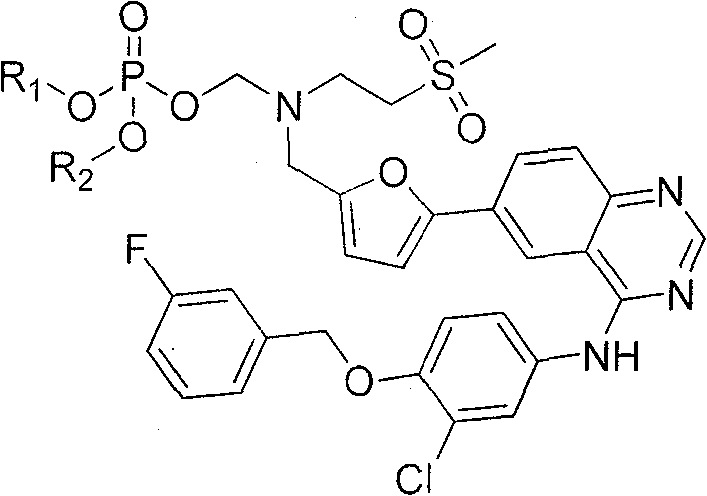
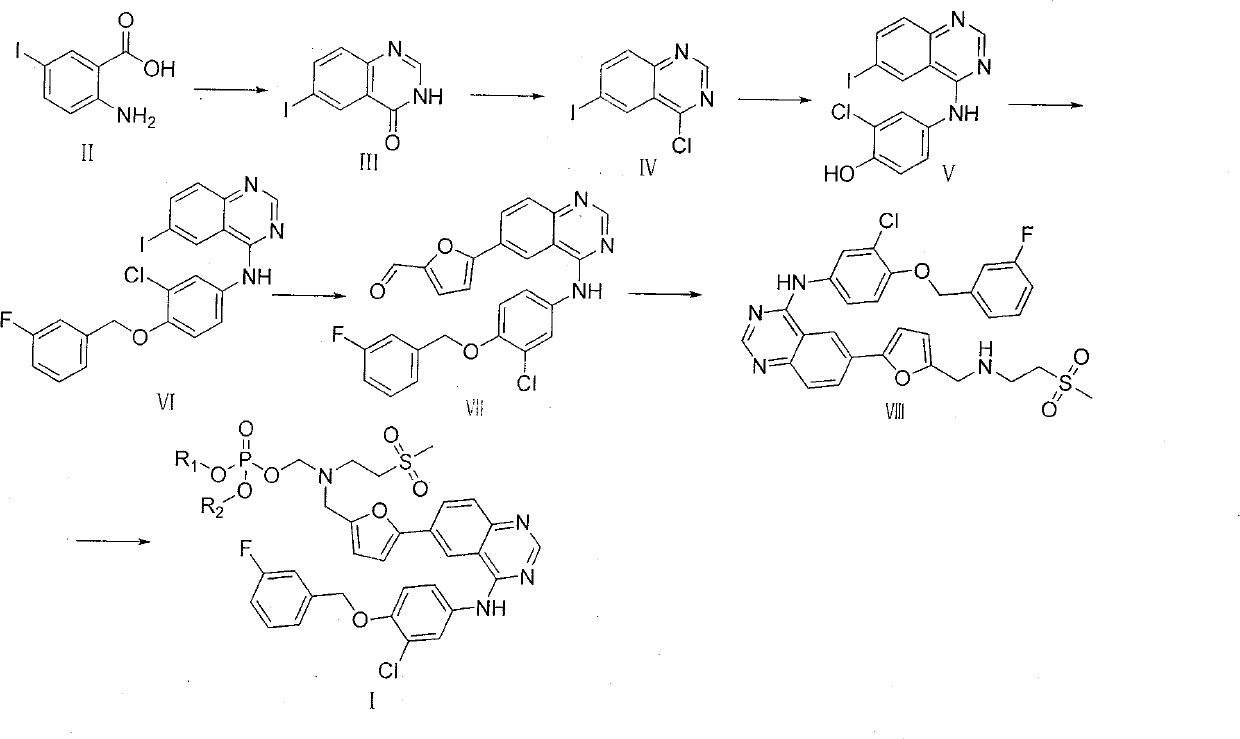
6-substituted quinazoline derivative, preparation method and application thereof
A quinazoline and derivative technology, applied in the field of medicinal chemistry, can solve the problems of increasing the difficulty of curing and the probability of recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 16-
[0024] The preparation of embodiment 16-iodoquinazolin-4-ketone III
[0025] 2-Amino-5-iodobenzoic acid (105.2g, 0.40mol) and methyl imidium acetate (83.2g, 0.80mol) were added to 1000mL of glycerol, heated to dissolve, and a large amount of white solids precipitated after half an hour. Cool and filter to obtain 93.5 g of white solid 6-iodoquinazolin-4-one III, yield 86.1%, m.p.135-136°C; 1 H NMR (400MHz, DMSO): δ7.43-8.37 (m, 4H, ArH), 12.36 (s, 1H, ArH). ESI-MS: m / z 273 [M+H] + .
Embodiment 24-
[0026] The preparation of embodiment 24-chloro-6-iodoquinazoline IV
[0027] Dissolve 6-iodoquinazolin-4-one (93.5g, 0.34mol) in DMF (300mL), add thionyl chloride dropwise, and heat to reflux for 3h. The solvent was evaporated, and ethyl acetate was recrystallized to obtain 4-chloro-6-iodoquinazoline IV (90.2 g, 90.5%) as a white solid, m.p.165-167°C; 1 H NMR (400MHz, DMSO): δ7.43-8.37 (m, 4H, ArH). ESI-MS: m / z 291 [M+H] + .
Embodiment 3
[0028] The preparation of embodiment 32-chloro-4-(6-iodoquinazoline-4-amino)phenol V
[0029] 4-Chloro-6-iodoquinazoline (90.2 g, 0.31 mol) and 4-amino-2-chlorophenol (44.5 g, 0.31 mol) were dissolved in isopropanol (500 mL), and heated to reflux for 2 h. After the solution was cooled, it was filtered, washed with isopropanol and ether, and dried to obtain 2-chloro-4-(6-iodoquinazoline-4-amino)phenol V (112.8g, 91.3%), m.p.143-144°C; 1 H NMR (400MHz, DMSO): δ4.03 (s, 1H, NH), 7.43-8.37 (m, 7H, ArH), 10.31 (s, 1H, OH). ESI-MS: m / z 398 [M+ H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com