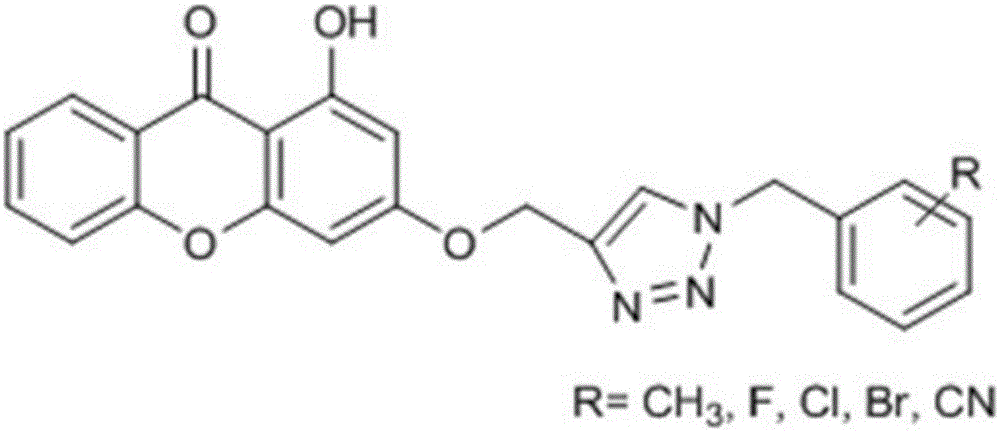
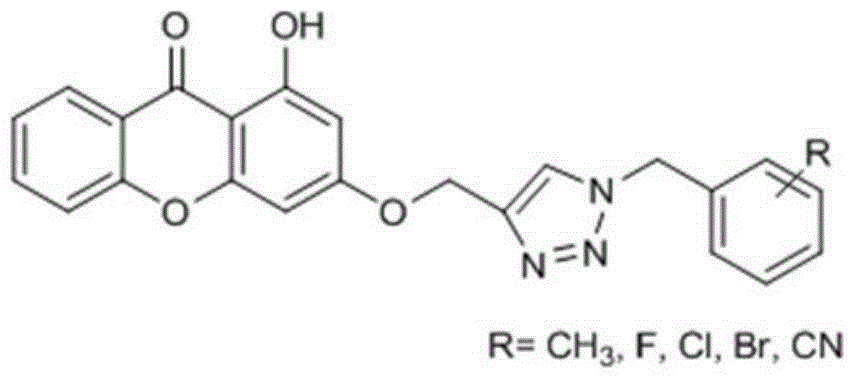
1,2,3-triazole substituted diphenyl pyrone compounds, preparation method and applications
A biphenylpyrone and compound technology, applied in the field of medicine, can solve problems such as structure and activity that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis of embodiment 1 compound of the present invention
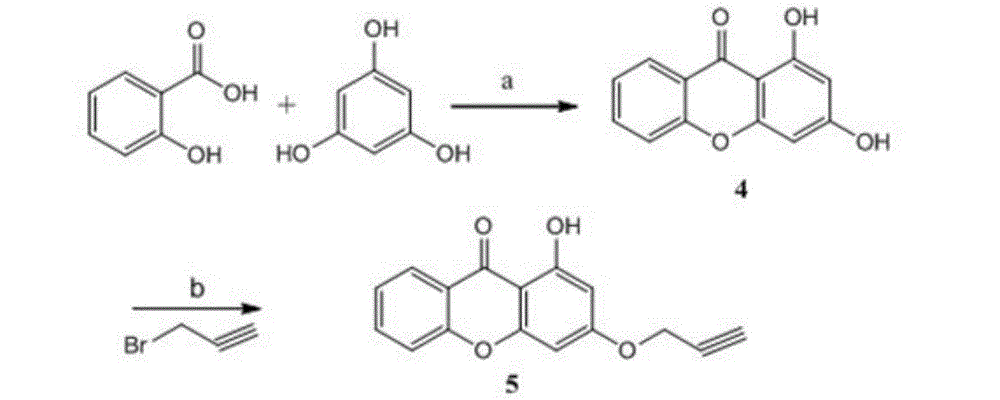
[0040] The preparation reaction scheme of compound of the present invention is as follows:
[0041]
[0042] Scheme Reagents and conditions: (a)P 2 o 5 / CH 3 SO 3 H85℃, 2.5h; (b) Propargylbronide, L 2 CO 3 , SMF, 4h; (c) Correspondingbenzylazide, DMSO, CuSO 4 .5H 2 O, rt, 16h.
[0043] The specific preparation method of the compound involved in the described reaction scheme route is as follows:
[0044] (1) Synthesis of 1,3-dihydroxybisphenylpyrone (4)
[0045] 2-Hydroxysalicylic acid (6.9g, 0.05mol) and phloroglucinol (6.3g, 0.05mol) were dissolved in Eaton's reagent, stirred and reacted at 85°C for 4 hours, the reaction was complete, cooled to room temperature, and an appropriate amount of ice water was added Excessive acid was quenched, stirred for 2 hours and left to stand, filtered, dissolved in ethyl acetate, washed with water until neutral, dehydrated with anhydrous sodium sulfate, eva...
Embodiment 2
[0055] The pharmacological experiment of embodiment 2 compounds of the present invention
[0056] (1) Experimental materials
[0057] 1. Test sample
[0058] After the target compounds were dissolved in DMSO (Merck), PBS (-) was added to make a 1000 μg / ml solution or a homogeneous suspension, and then diluted with DMSO-containing PBS (-).
[0059] 2. Cell lines
[0060] A549 (human non-small cell lung cancer cells).
[0061] 3. Other materials and main instruments
[0062] Culture medium: RPMI1640 + 15% NBS + double antibody; MTT: [thiazolyl blue] was purchased from Sigma Company (St.Louis, MO, USA); other reagents were domestic analytical grade. Fully automatic microplate reader WellscanMK-2 (Labsystems company), CO 2 Constant temperature incubator (Japan Sanyo Company), imported 96-well culture plate, etc.
[0063] 4. Experimental method
[0064] MTT (tetrazolium salt) method: 96-well plate per hole concentration is 4 ~ 6 × 10 4 cells / ml cell suspension 100 μl, set a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com