Super fast-acting insulin compositions
A technology of effective insulin and composition, applied in the field of super fast-acting insulin composition, which can solve problems such as obesity, prolonged action time, and hypoglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
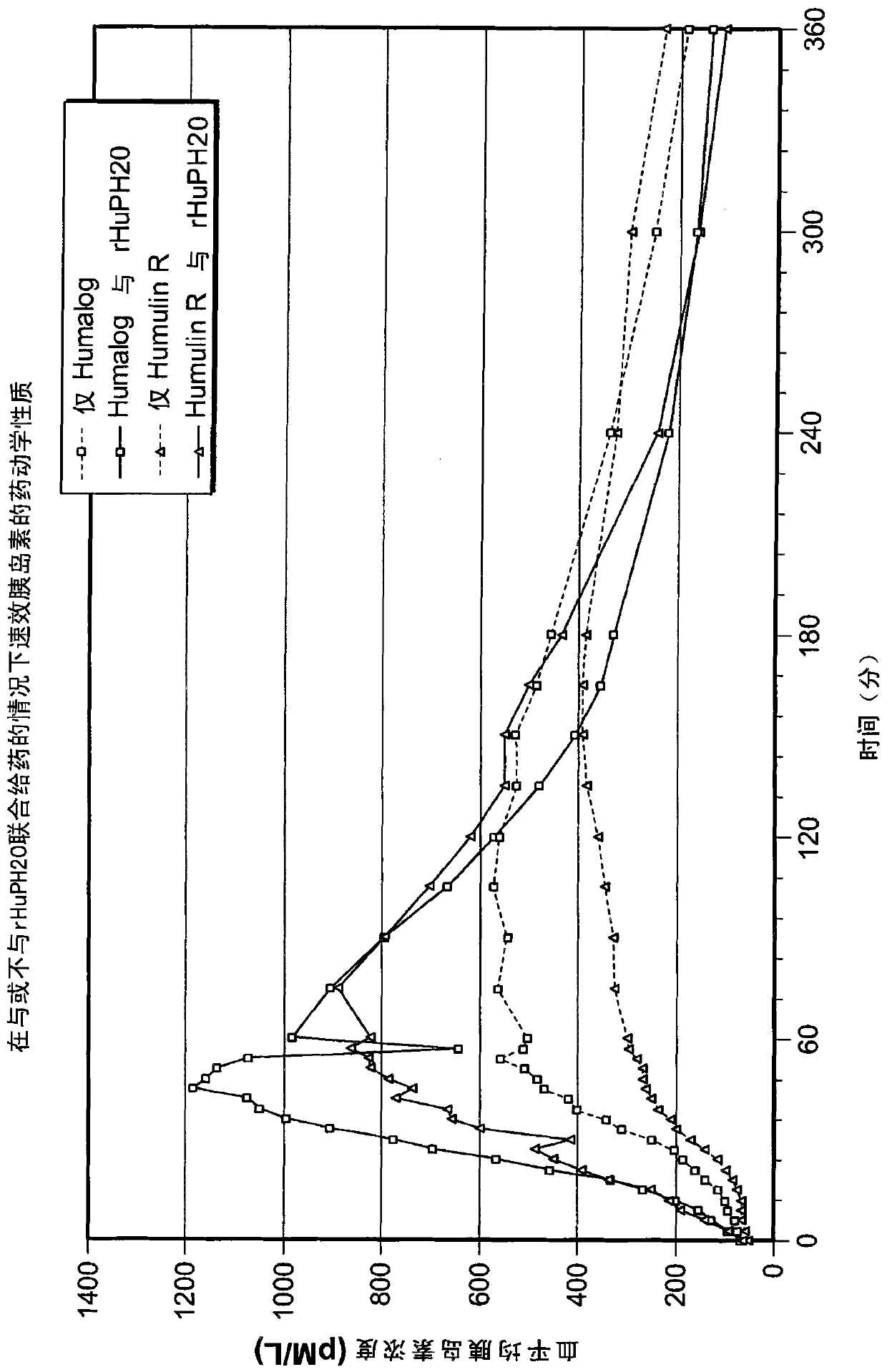
[0457] Co-administration of recombinant human PH20 (rHuPH20) and rapid-acting insulin contributes to improved pharmacokinetics and pharmacodynamics
[0458] Insulin, including insulin analogs, is administered to individuals with diabetes for the management of hyperglycemia. To more effectively replicate the normal physiological prandial insulin release observed in healthy individuals, a clinical study was conducted to determine whether co-administration of recombinant human PH20 (rHuPH20) could increase the early absorption rate and absorption of administered rapid-acting insulin quantity. Increased absorption may result in a more accelerated acting of the rapid-acting insulin and thus more closely mimic the endogenous insulin-time profile observed in healthy individuals. This may provide clinical benefits in terms of better glycemic control and reduced weight gain in diabetic individuals. The clinical study was designed to evaluate rHuPH20 when administered subcutaneously a...
Embodiment 1a
[0460] With or without co-administration of rHuPH20 R insulin and Pharmacokinetics and Pharmacodynamics of Insulin Lispro in Healthy (Non-diabetic) Individuals
[0461] A randomized, double-blind, crossover, two-stage, continuous controlled study (2-arm study) was conducted to evaluate subcutaneous administration of 20 units of insulin lispro or R insulin. Twenty-five healthy adult male subjects were enrolled in this study. In Phase 1, 12 individuals received Subcutaneous injection of insulin lispro and rHuPH20 and only A separate subcutaneous injection of insulin lispro. Injections are usually 7 days apart, with half the individuals receiving the first Insulin lispro and rHuPH20, followed by separate Insulin lispro, half of the individuals first received a separate insulin lispro, then receive Insulin lispro and rHuPH20. In Phase 2, 13 individuals received Subcutaneous injection of R insulin and rHuPH20 and only A separate subcutaneous injection of in...
Embodiment 1b
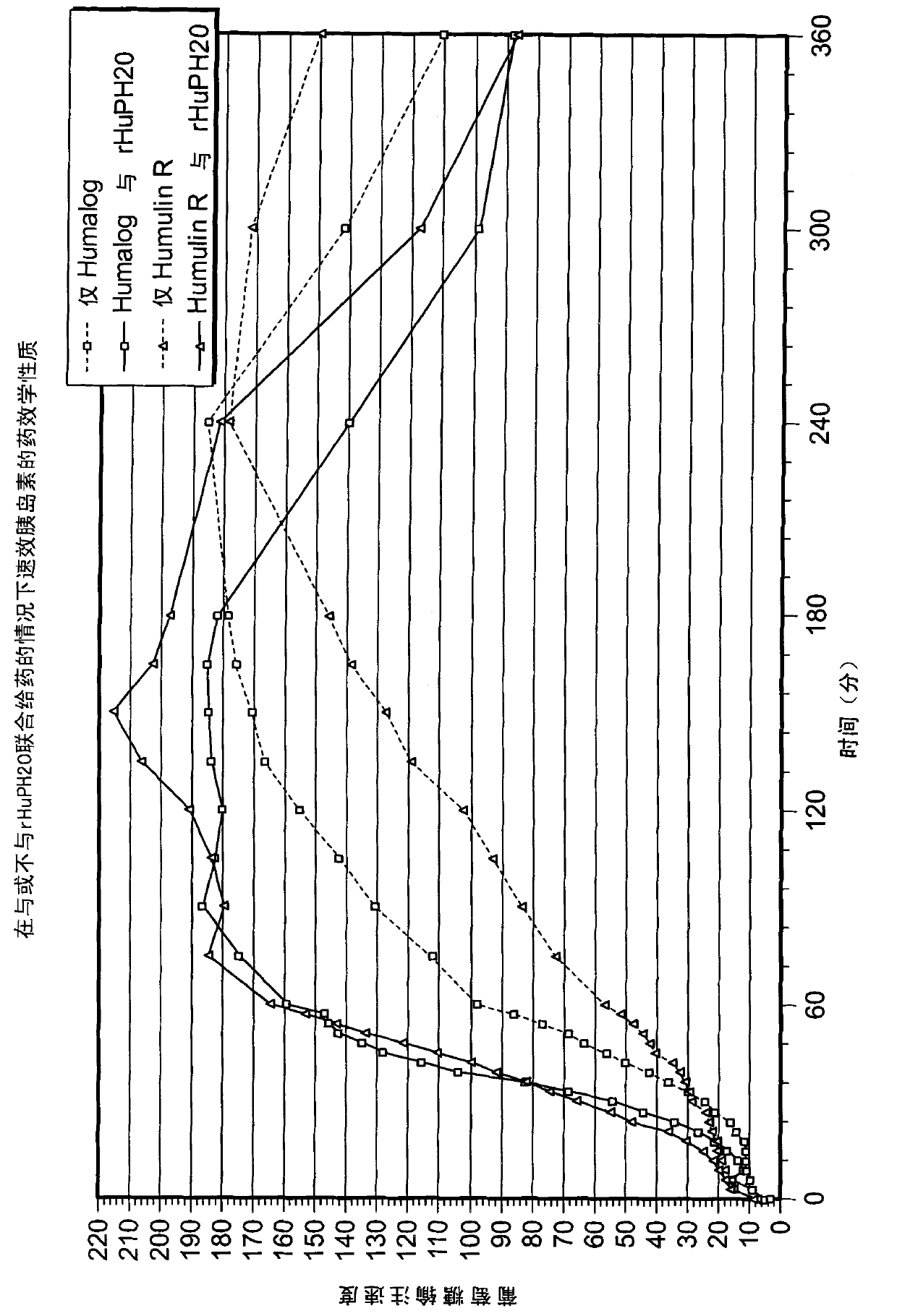
[0555] Subcutaneously administered with and without rHuPH20 insulin lispro or Pharmacokinetics and postprandial glycemic response of R insulin in patients with type 1 diabetes mellitus after eating liquid food
[0556] The efficacy of subcutaneous injection with and without rHuPH20 co-administration was evaluated insulin lispro and A study of the pharmacokinetics and postprandial glycemic response (i.e. pharmacodynamics (PD)) of R insulin in type 1 diabetic patients following a liquid diet. The trial was a single-blind (only patients blinded), single-centre, crossover, liquid-food trial (including a series of standardized liquid-food loads) in patients with type 1 diabetes mellitus, with blood samples collected 2 hours before and 8 hours after dosing. In the analysis of PK and PD parameters.
[0557] Each individual experiences a series of A dose-finding visit of insulin lispro and rHuPH20 (visits 2A-C; a total of three injections) to determine optimal glycemic contr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com