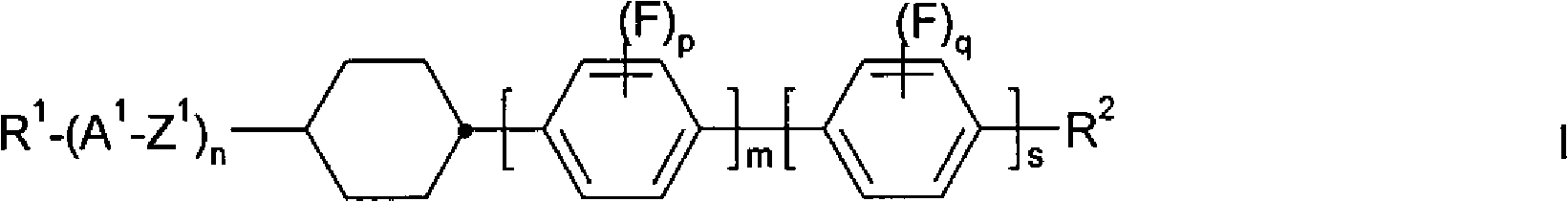
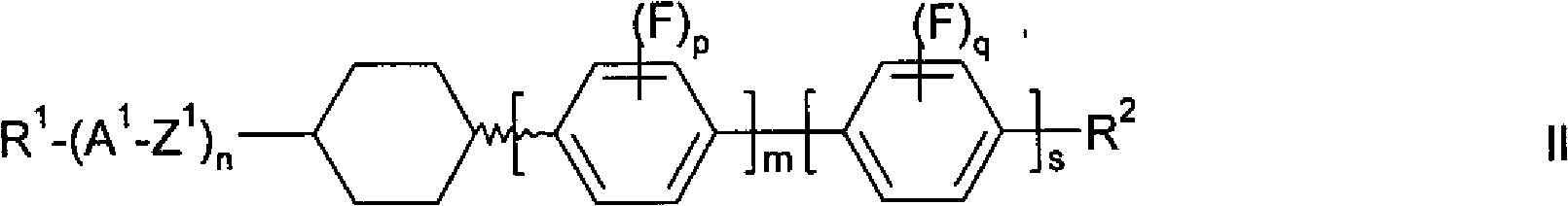
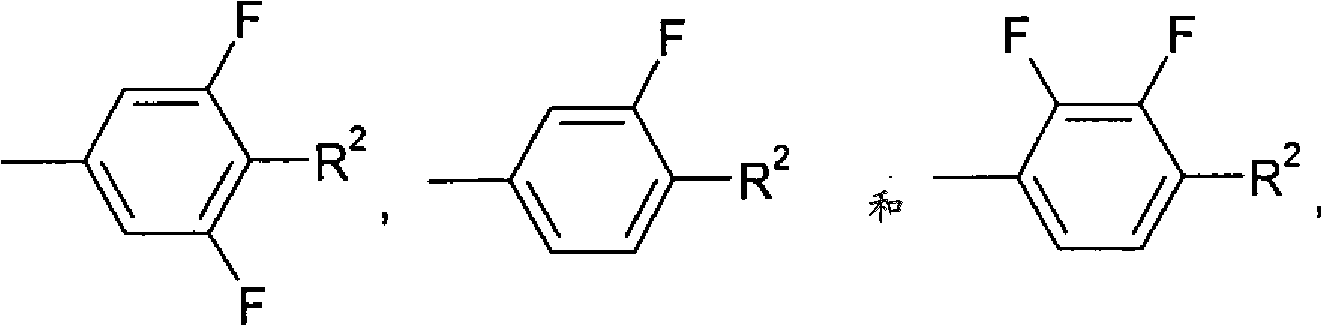
Isomerization method
A technology of compounds, carbon atoms, applied in the field of isomerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]
[0059] 20 g of starting material (64 mmol, 44% cis on central ring) were dissolved in 90 g of anhydrous dichloromethane (for analysis) and cooled to -45°C. 2 g of trifluoromethanesulfonic acid (13 mmol, 1.2 ml) were added to the suspension. Then 0.15 g of 1-adamantanol (1.0 mmol) in 9 g of dichloromethane was added dropwise. The batch was stirred at -40 to -45°C for an additional 1 to 3 hours, with occasional samples taken to measure the degree of isomerization. When isomerization was complete (sample was completely isomerized after 1-2 hours), the batch was processed.
[0060] For work-up, 38 ml of water were added to the batch, which was then warmed to about 20°C. The organic phase was separated off, washed with 22 g of trisodium citrate solution (25% in water) and 2×20 ml of water and evaporated to dryness in a rotary evaporator. The residue (20.2 g) was taken up in heptane and evaporated again. The crude product (pale yellow crystals) had a cis / trans conten...
Embodiment 2
[0063]
[0064] 168 g of starting material (525 mmol, 59.3% cis on central ring) were dissolved in 650 ml of anhydrous dichloromethane (for analysis) and cooled to -40 / -45°C. 9.5 ml of trifluoromethanesulfonic acid (108 mmol) were added to the suspension. 1.26 g of 1-adamantanol (8.3 mmol) in 50 ml of dichloromethane were then added dropwise. The batch was stirred at -40 to -45°C for an additional 1 to 2 hours, with occasional samples taken to measure the degree of isomerization. When isomerization was complete (sample was completely isomerized after 30 minutes), the batch was processed.
[0065] For work-up, a mixture of 175 g of ice and 350 ml of 25% hydrochloric acid was added to the batch, which was then warmed to about 20°C. The organic phase is separated off, washed with 175 ml of sodium bicarbonate solution (5% in water) and 175 ml of water and evaporated to dryness in a rotary evaporator. The residue (168.8 g) was taken up in heptane. The crude product (pale yel...
Embodiment 3
[0068] Example 1 was repeated while exchanging the catalyst 1-adamantanol for an equivalent amount of 2-chloro-2-methylpropane.
[0069] The crude product (pale yellow crystals) had 1.1% cis / trans product content, and 97.9% desired trans / trans product content (HPLC).
[0070] Other combinations of embodiments and variants of the invention can be derived from the claims.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com