A solid pharmaceutical formulation
A formulation and drug technology, applied in the field of sustained-release solid pharmaceutical formulations, can solve the problems of not so good drug release performance and reducing the firmness of solid formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
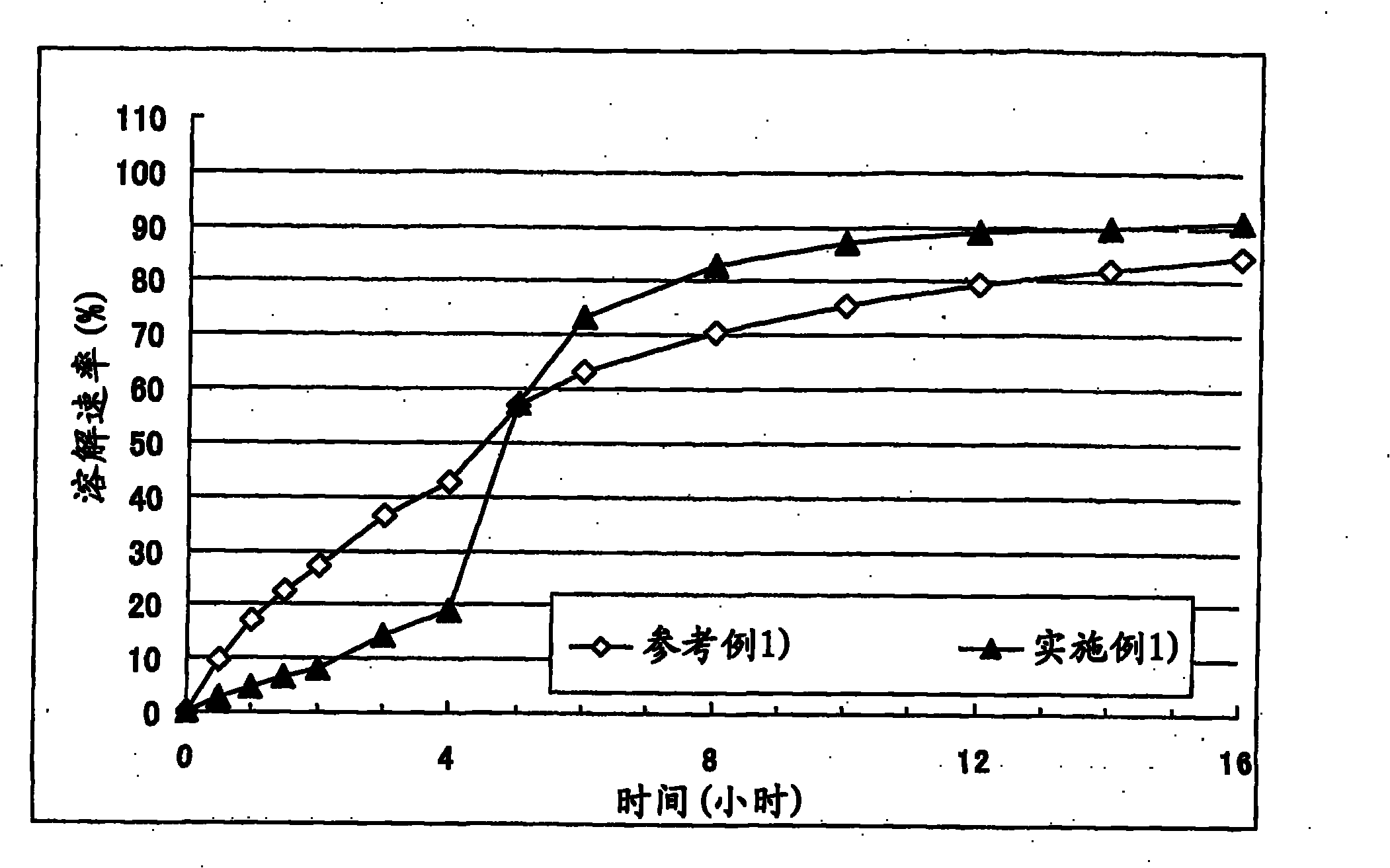
Embodiment 1
[0069] Corn starch (trade name: "Nisshoku Corn Starch", manufactured by Nippon Shokuhin Kako K.K.) (90 g), crystalline cellulose (trade name: "CEOLUS PH301", manufactured by Asahi Kasei Corporation) (30 g), hydroxypropyl acetate Athylmethylcellulose succinate (trade name: "AS-HF", manufactured by Shin-Etsu Chemical Co., Ltd.) (30 g) and cilostazol (manufactured by Otsuka Pharmaceutical Co., Ltd.) (150 g ) was mixed, the mixture was put into an accelerated kneader (model: NSK-150, manufactured by Okada Seiko K.K.), and then 37.5 mL of 4% polysorbate 80 aqueous solution (containing 1.5 g Polysorbate 80) and purified water (92.5 g) to obtain a starting composition (starting composition example 1).
[0070] The starting composition was subjected to extrusion granulation with an extrusion granulator equipped with a dome die (hole diameter: 0.6 mm, DomeGran DG-L1, manufactured by DALTON Co. LTD.) to obtain wet granules. The wet granules were treated with a spheroidizer (Murmerizer ...
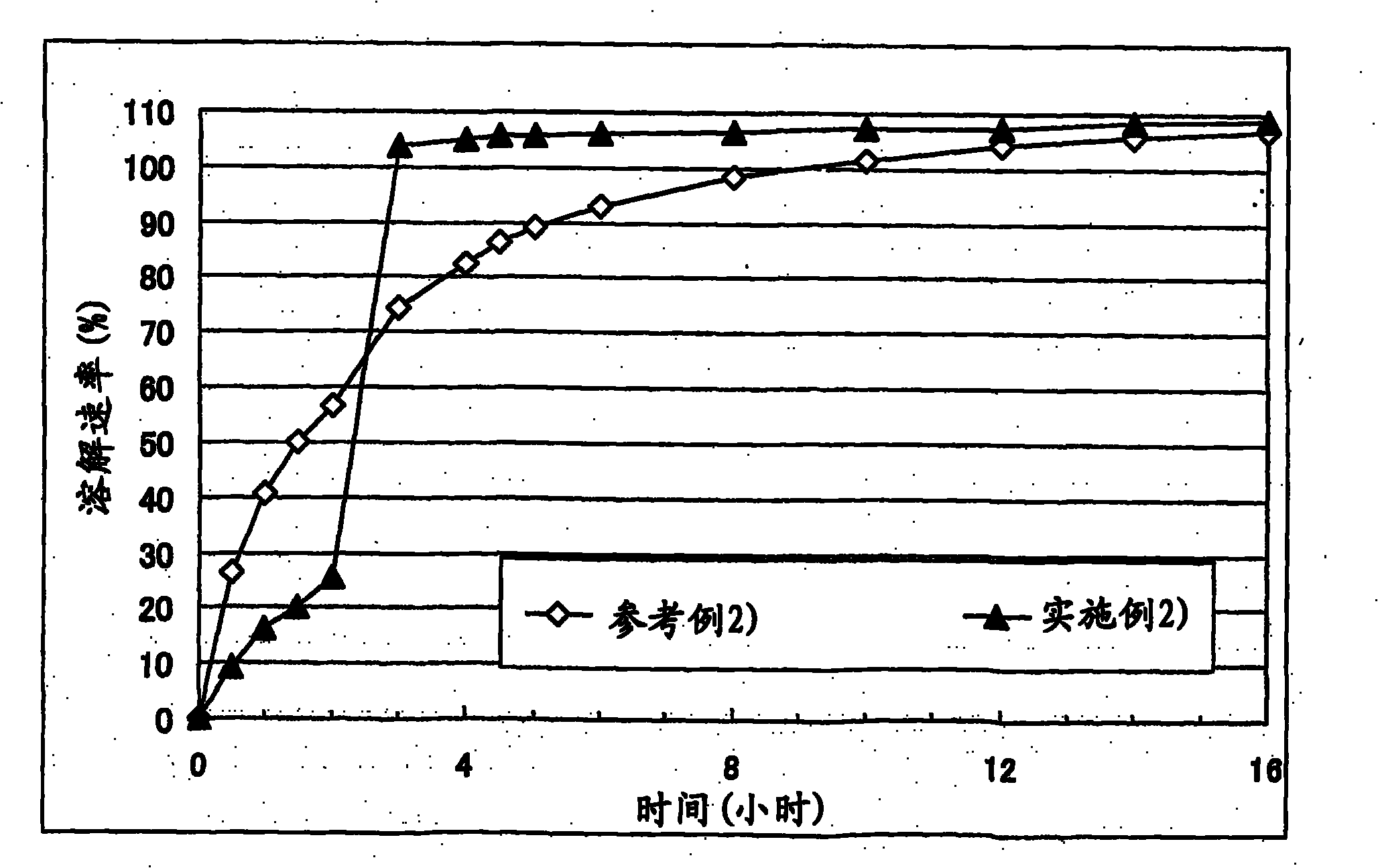
Embodiment 2
[0073] Corn starch (trade name: "Nisshoku Corn Starch", manufactured by Nippon Shokuhin Kako K.K.) (90 g), crystalline cellulose (trade name: "CEOLUS PH301", manufactured by Asahi Kasei Corporation) (30 g), hydroxypropyl acetate Athylmethylcellulose succinate (trade name: "AS-HF", manufactured by Shin-Etsu Chemical Co., Ltd.) (30 g) and cilostazol (manufactured by Otsuka Pharmaceutical Co., Ltd.) (150 g ) was mixed, the mixture was put into an accelerated kneader (model: NSK-150, manufactured by Okada Seiko K.K.), and then 37.5 mL of 4% polysorbate 80 aqueous solution (containing 1.5 g Polysorbate 80) and purified water (67.5 g) to obtain a starting composition (starting composition example 2).
[0074] The starting composition was subjected to extrusion granulation with an extrusion granulator equipped with a dome die (hole diameter: 0.6 mm, DomeGran DG-L1, manufactured by DALTON Co. LTD.) to obtain wet granules. The wet granules were treated with a spheroidizer (Murmerizer ...
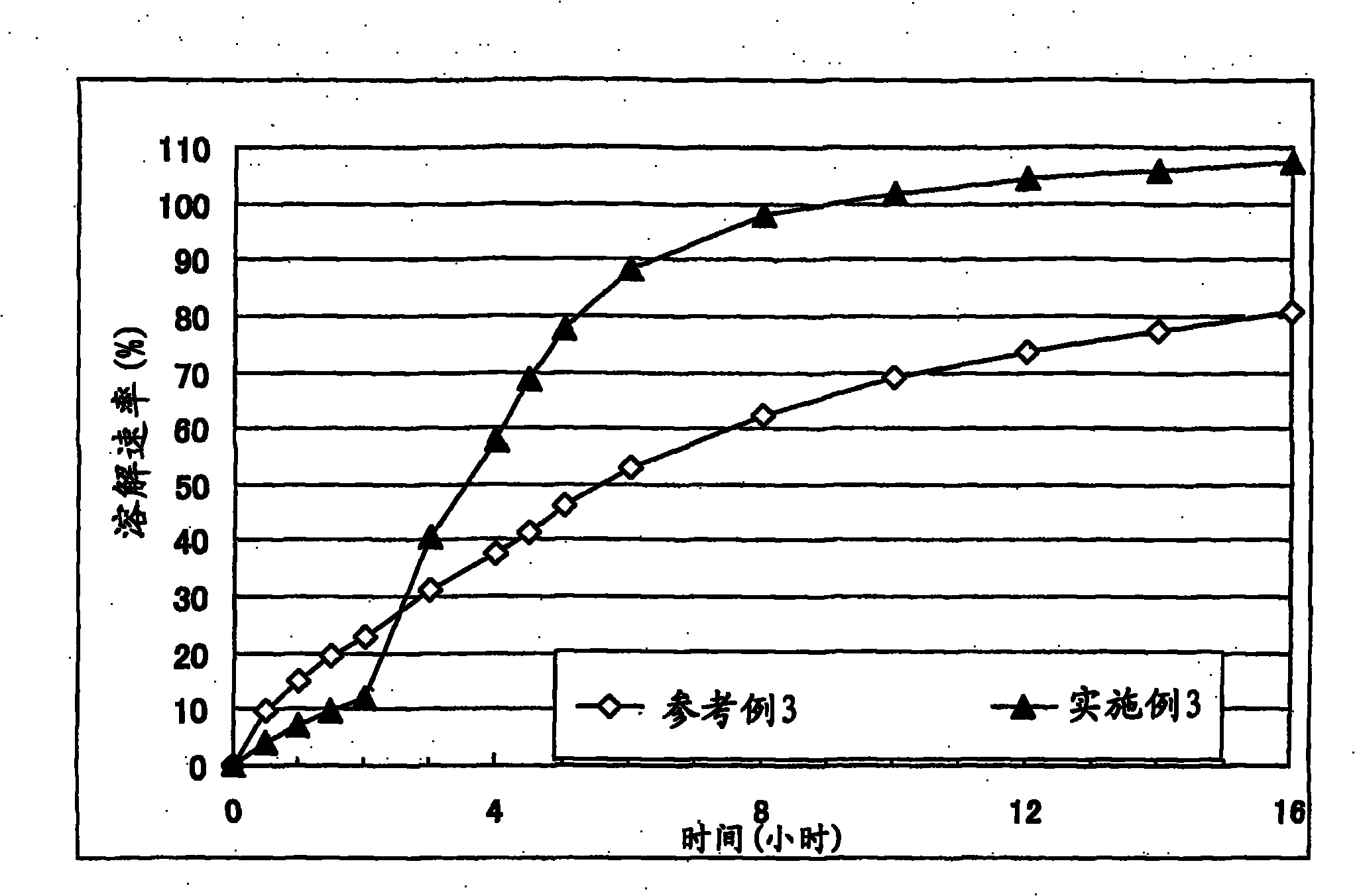
Embodiment 3
[0077] Corn starch (trade name: "Nisshoku Corn Starch", manufactured by Nippon Shokuhin Kako K.K.) (69 g), crystalline cellulose (trade name: "CEOLUS PH301", manufactured by Asahi Kasei Corporation) (51 g), methacrylic acid Copolymer L (trade name: "Eudragit L100", manufactured by EVONIK) (30 g) and cilostazol (manufactured by Otsuka Pharmaceutical Co., Ltd.) (150 g) were mixed, and the mixture was put into an accelerating kneader (model: NSK-150, manufactured by Okada Seiko K.K.), and purified water (130 g) was added thereto with stirring to obtain a starting composition (starting composition example 3).
[0078] The starting composition was subjected to extrusion granulation with an extrusion granulator equipped with a dome die (hole diameter: 0.6 mm, DomeGran DG-L1, manufactured by DALTON Co. LTD.) to obtain wet granules. The wet granules were treated with a spheroidizer (Murmerizer QJ-400, manufactured by DALTON Co. LTD.) to adjust the shape and size of the granules, there...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com