Gene treatment medicament capable of inhabiting immunoreaction caused by transgenosis products and preparation method thereof
A gene drug and immune response technology, applied in gene therapy, drug combination, pharmaceutical formula, etc., can solve the problem of no solution for cellular immune response, achieve the effect of reducing cellular immune toxicity and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of rAAV / OVA-155T
[0021] ① Design of miR-155 tight binding sequence 155T
[0022] The tight binding sequence 155T: 5'-CCCCTATCACAATTAGCATTAA-3' was designed referring to the miR-155 sequence published on the relevant website (eg www.mirbase.org).
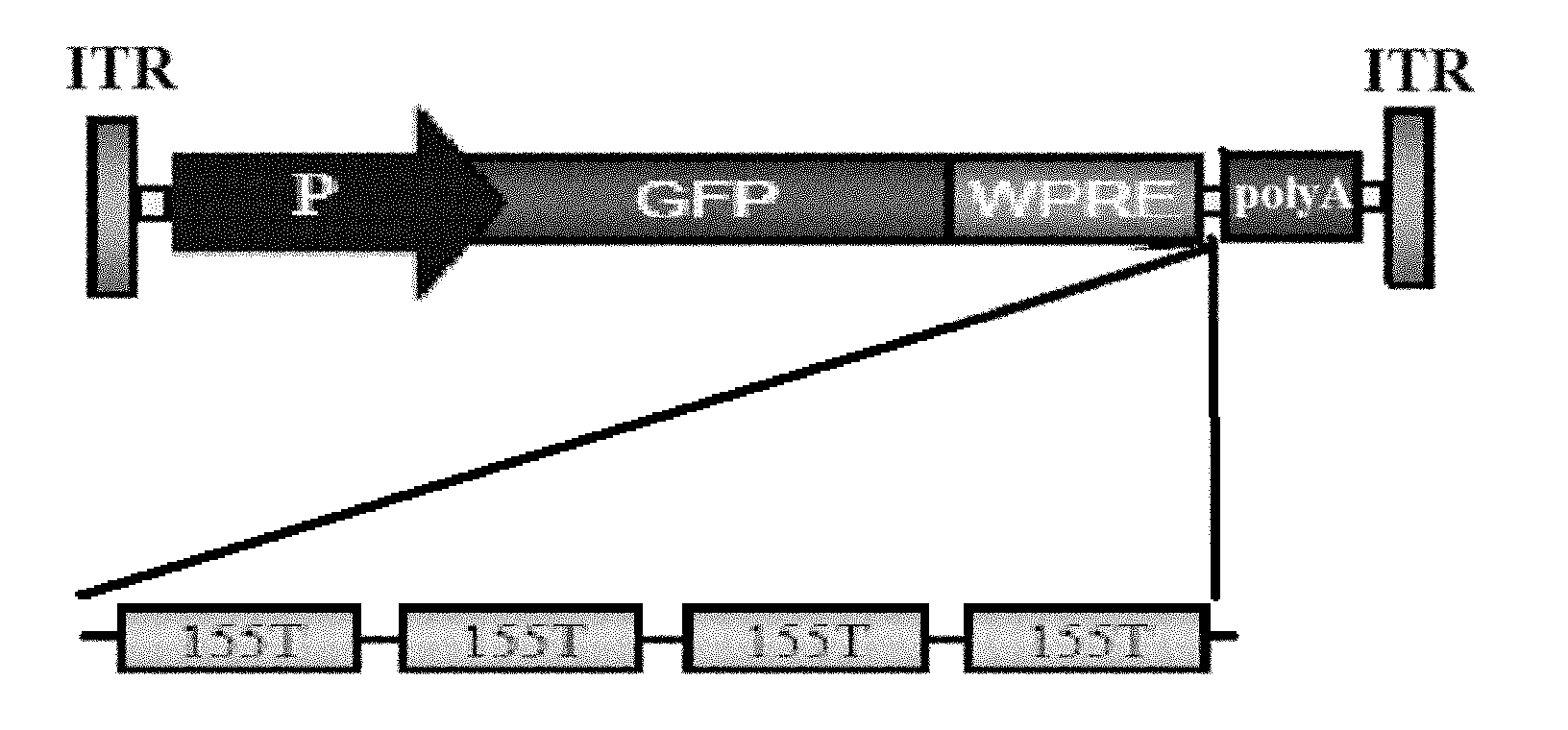
[0023] ② Construction of vector plasmid pAAV-GFP-155T
[0024] Design four tandem 155T sequences as: 5'-TCTAGAGTCG
[0025] CGAT CGGT GTGA CTCGAG-3'. In the box is the tight binding sequence 155T.
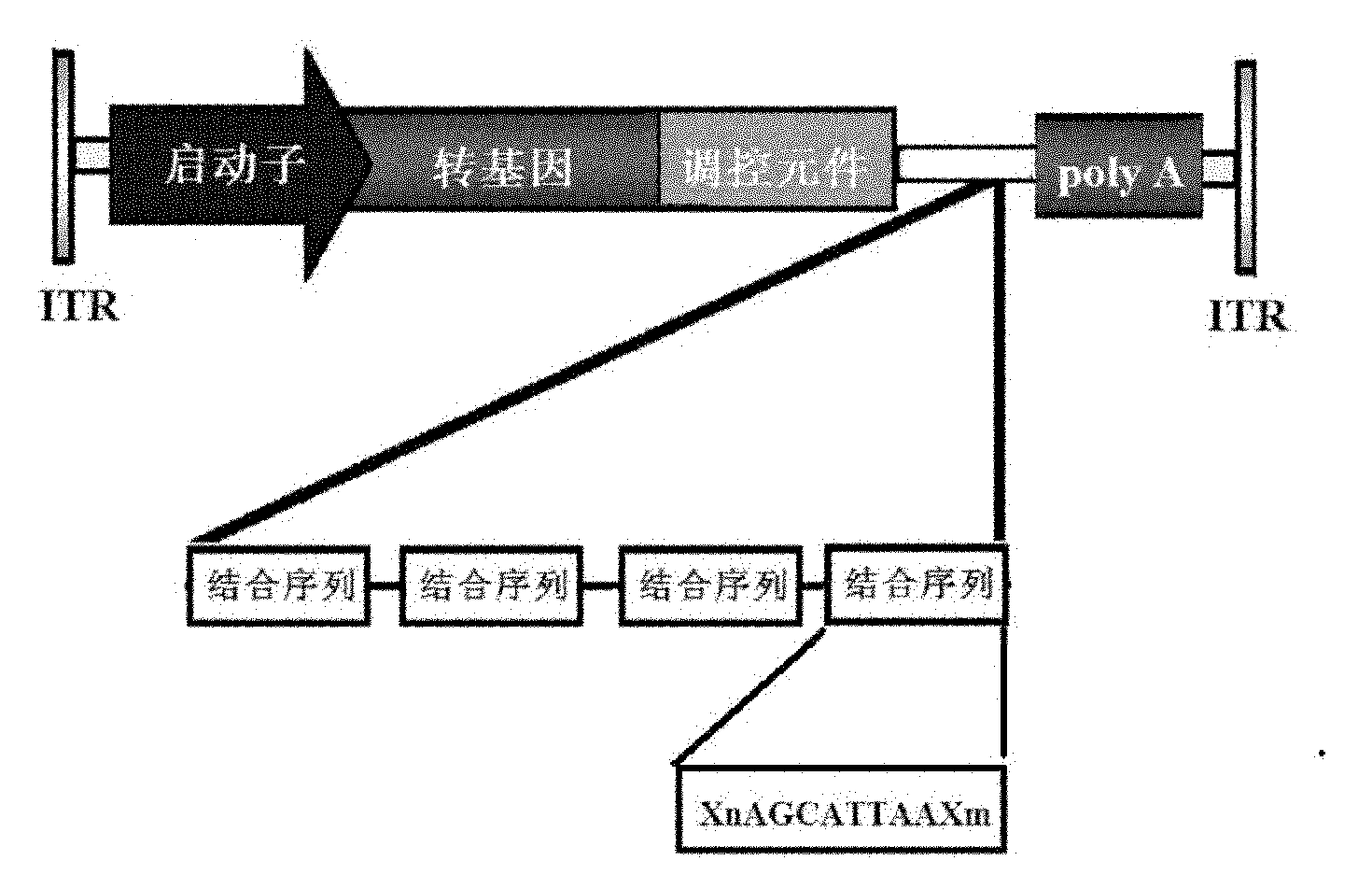
[0026] After the above sequence is double-digested with XhoI and XbaI, it is connected with the same double-digested rAAV vector plasmid, (the gene expression cassette of the vector plasmid contains promoter, transgene, transgene expression regulatory element, MicroRNA binding sequence, polyA Tail, AAV2 ITR (inverted terminal repeat sequence) at both ends of the gene expression frame, see figure 1 shown); the vector plasmid pAAV-GFP-155T was obtained. In pAAV-GFP-155T, four 155T sequences are connected i...
Embodiment 2
[0032] Preparation of rAAV / OVA-155TN
[0033] ① Design of miR-155 non-tight binding sequence 155TN
[0034] Only 8 bases at the 3' end of the miR-155 tight binding sequence 155T in Example 1 were retained, and the remaining bases were randomly changed to obtain the miR-155 non-tight binding sequence 155TN: 5'-XmAGCATTAA-3'. Where X represents a random base, and m is the number of random bases. In this embodiment, ATCCATCGACCGAA is used for Xm, and m is 14.
[0035] ② Construction of vector plasmid pAAV-GFP-155T
[0036] According to the method of Example 1, the four 155TN sequences were connected in series to the 3'UTR region of the rAAV vector plasmid transgene expression cassette, (the gene expression cassette of the vector plasmid contains a promoter, a transgene, and a transgene expression regulatory element in sequence , MicroRNA binding sequence, polyA tail, AAV2 ITR (inverted terminal repeat sequence) at both ends of the gene expression frame, see figure 1 shown); the...
Embodiment 3
[0042] Preparation of rAAV / OVA-155TN(1) and rAAV / OVA-155TN(8)
[0043] ①Construction of vector plasmids pAAV-GFP-155TN(1) and pAAV-GFP-155TN(8)
[0044] According to the method of Example 1, one 155TN sequence or eight 155TN sequences were connected in series to the 3'UTR region of the rAAV vector plasmid transgene expression cassette, (the gene expression cassette of the vector plasmid contains promoter, transgene, Transgene expression regulatory elements, MicroRNA binding sequence, polyA tail, AAV2 ITR (inverted terminal repeat sequence) at both ends of the gene expression frame, see figure 1 shown); the vector plasmids pAAV-GFP-155TN (1) and pAAV-GFP-155TN (8) were obtained respectively.
[0045] ②Construction of vector plasmids pAAV-OVA-155TN(1) and pAAV-OVA-155TN(8)
[0046] Ovalbumin (OVA) cDNA was used instead of GFP cDNA to obtain vector plasmids pAAV-OVA-155TN(1) and pAAV-OVA-155TN(8), respectively.
[0047] ③ Preparation of rAAV-OVA-155TN (1) and rAAV-OVA-155TN (8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com