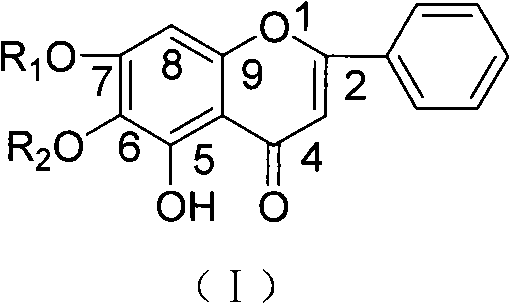
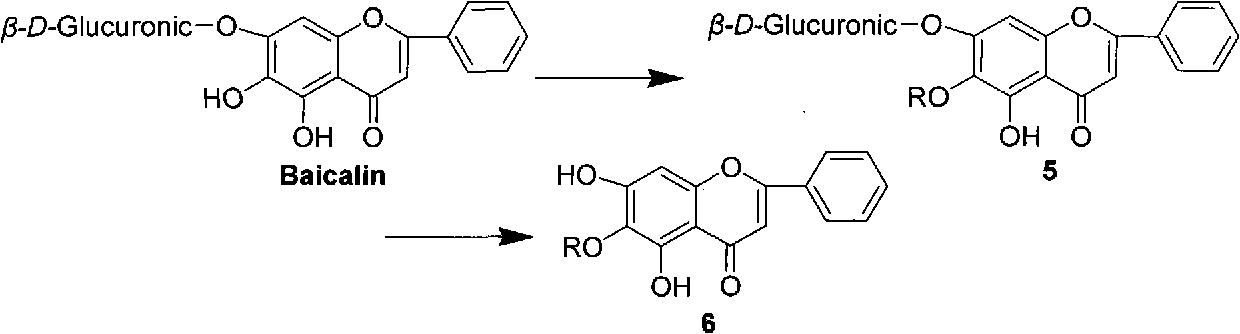
Baicalein derivatives and preparation method and application thereof
A technology of baicalein and derivatives is applied in the application field of prevention and treatment of chronic obstructive pulmonary emphysema, and achieves the effects of low cost, low price and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 16
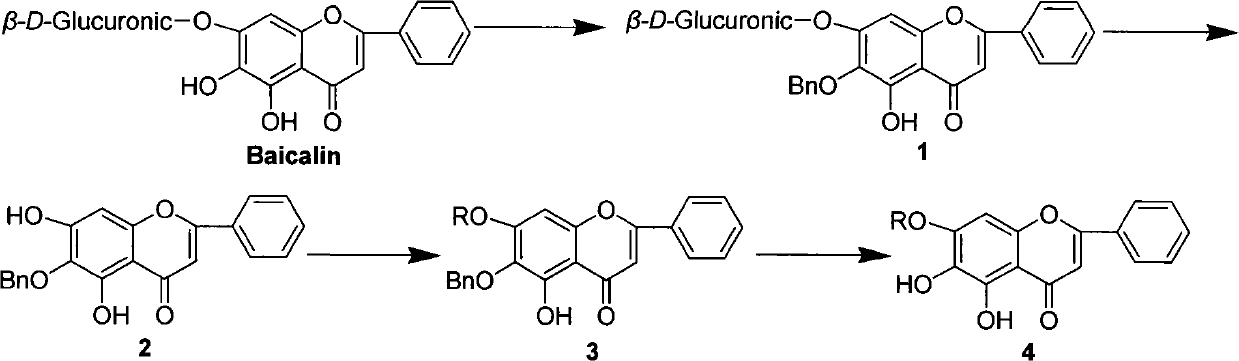
[0032] The preparation of embodiment 16-benzyloxybaicalein
[0033]
[0034] Dissolve 13.38g (30mg) baicalin in 50ml DMF, add anhydrous K 2 CO 3 20.76g (150mmol), 3.56ml (30mmol) of benzyl bromide, and 300ml of anhydrous acetone were placed in a 500ml reaction bottle, and refluxed in a dry state until the reaction was complete, then filtered while hot. After the filtrate was evaporated to dryness under reduced pressure, adjust the pH to 5 with 10% acetic acid solution, collect the solid, add 200ml of ethanol and 20ml of concentrated HCl into a 500ml reaction bottle, reflux until the reaction is complete, evaporate the solvent under reduced pressure, and wash the impurities with water. After that, use CH 2 Cl 2 -EtOH was repeatedly recrystallized to obtain 5.69g, and the total yield of the two steps was 52.6%.
[0035] 1 H-NMR (600MHz, DMSO): δ8.04 (2H, m), 7.52-7.61 (3H, m), 7.43 (4H, m), 7.35 (1H, m), 6.87 (1H, s), 6.62 ( 1H, s), 4.82 (2H, s); ESI-MS m / z: 361 [M+H] ...
Embodiment 26
[0036] The preparation of embodiment 26-n-butoxybaicalein
[0037]
[0038] Dissolve 4.46g (10mmol) baicalin in 25ml DMF, add anhydrous K 2 CO 36.92g (50mmol), 1.07ml (10mmol) of 1-bromo-n-butane, 150ml of anhydrous acetone, placed in a 250ml reaction flask, refluxed in a dry state, until the reaction was complete, filtered, evaporated to dryness, and dissolved in 10% The acetic acid solution adjusted the pH to 5, collected the solid, added to a 250ml reaction flask containing 150ml of ethanol and 10ml of concentrated HCl, refluxed until the reaction was complete, filtered, and column chromatographed to obtain 1.57g of a light yellow solid, with a yield of 48.1%.
[0039] 1 H-NMR (600MHz, DMSO): δ8.04 (2H, m), 7.54-7.60 (3H, m), 6.93 (1H, s), 6.61 (1H, s), 3.94 (2H, m), 1.66 ( 2H, m), 1.44 (2H, m), 0.89 (3H, m); ESI-MS m / z: 327 [M+H] + , 349[M+Na] + (pos i t ive mode).
Embodiment 36
[0040] The preparation of embodiment 36-allyloxybaicalein
[0041]
[0042] Dissolve 4.46g (10mmol) baicalin in 25ml DMF, add anhydrous K 2 CO 3 6.92g (50mmol), 0.86ml (10mmol) of allyl bromide, 150ml of anhydrous acetone, placed in a 250ml reaction bottle, refluxed in a dry state, until the reaction was complete, filtered while hot, evaporated to dryness, and dissolved in 10% acetic acid The pH of the solution was adjusted to 5, the solid was collected, added to a 250ml reaction bottle containing 150ml of ethanol and 10ml of concentrated HCl, refluxed until the reaction was complete, filtered, and column chromatography gave 1.74g of a light yellow solid, with a yield of 56.2%.
[0043] 1 H-NMR (600MHz, DMSO): δ8.06(2H, m), 7.55-7.62(3H, m), 7.01(1H, s), 6.95(1H, s), 6.03(1H, m), 5.17( 1H, m), 5.29 (1H, m), 4.48 (2H, m); ESI-MS m / z: 311 [M+H] + , 333[M+Na] + (positive mode).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com