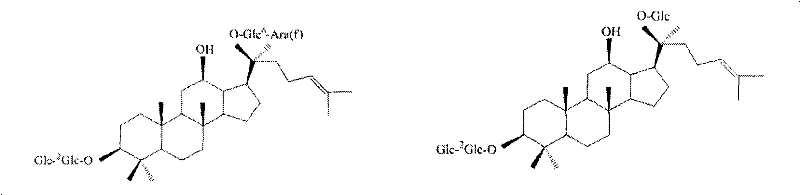
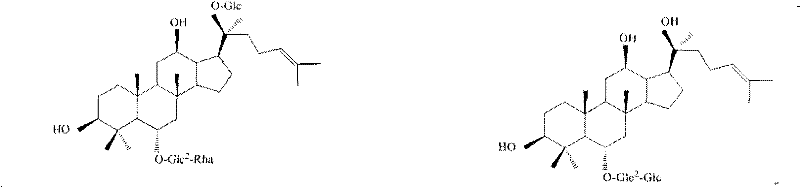Method for determining content of active compounds in Chinese medicinal freeze-dried injection
A technology of freeze-dried injection and determination method, applied in the field of pharmaceutical preparation analysis, can solve the problems of cumbersome, low content, time-consuming and the like, and achieve the effect of reliable quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Raw material weight ratio:
[0096] Total ginseng saponins extract 50g Epimedium total flavonoids extract 40g
[0097] Preparation:
[0098] a. Dissolve 800 grams of hydroxypropyl-β-cyclodextrin with 4000ml of water to make a 20% solution, add 750ml (5.33%) of total flavonoids extract of Epimedium and heat to dissolve, slowly add hydroxypropyl-β-cyclodextrin in stages - In the cyclodextrin 800 solution, keep warm at 80°C±1°C, dynamically stir for inclusion, and the inclusion time is 3 hours. Add 100ml (50%) of the total ginseng saponins extract to water and heat to dissolve, add it to the inclusion solution, and continue the inclusion 1 hour;
[0099] b. Add water to adjust the total amount of the solution to 6000ml, adjust the pH to 7.5 with 10% sodium hydroxide solution, add 24 grams of activated carbon (that is, contain 0.4% activated carbon), boil for 10 minutes, filter with a clarified plate while it is hot, and filter out the activated carbon , and then filter ...
Embodiment 2
[0115] Raw material weight ratio:
[0116] Total Ginseng Saponin Extract 35g Epimedium Total Flavonoid Extract 55g
[0117] Preparation:
[0118] a. Dissolve 900 grams of hydroxypropyl-β-cyclodextrin in 3000ml of water to make a 30% solution, add 1800ml (3.11%) of the total flavonoids extract of Epimedium to dissolve, and slowly add hydroxypropyl-β- In the cyclodextrin solution, keep warm at 50±1°C, dynamically stir for inclusion, and the inclusion time is 8 hours. Add 105ml (33.3%) of the total ginseng saponins extract to water and heat to dissolve, add it to the inclusion solution, and continue inclusion for 2 hours;
[0119] b, add water to adjust the total amount of the solution to 6000ml, adjust the pH to 6.2 with 20% sodium hydroxide solution, add 6 grams of gac (containing 0.1% gac), boil for 10 minutes, filter while hot with a sand filter stick, filter gac, Then filter with 0.22μm microporous membrane, and dilute to 6000ml with water;
[0120] c. Autoclave at 105°C ...
Embodiment 3
[0135] Raw material weight ratio:
[0136] Total Ginseng Saponin Extract 65g Epimedium Total Flavonoid Extract 25g
[0137] Preparation:
[0138] a. Dissolve 650 grams of hydroxypropyl-β-cyclodextrin with 4000 g of water to make a 16.3% solution, add 250 ml (10%) of the total flavonoids extract of Epimedium and heat to dissolve, slowly add hydroxypropyl-β-cyclodextrin in stages - In the cyclodextrin solution, keep warm at 90°C ± 1°C, stir dynamically for inclusion, and the inclusion time is 3 hours. Add 130ml (50%) of the total ginseng saponin extract to water and heat to dissolve, add it to the inclusion solution, and continue inclusion for 1 Hour;
[0139] b. Add water to adjust the total amount of the solution to 6000ml, adjust the pH to 8.5 with 5% sodium hydroxide solution, add 30 grams of activated carbon (containing 0.5% activated carbon), boil for 6 minutes, suction filter while it is hot, filter out the activated carbon, and then use 0.22 Filter with μm microporous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com