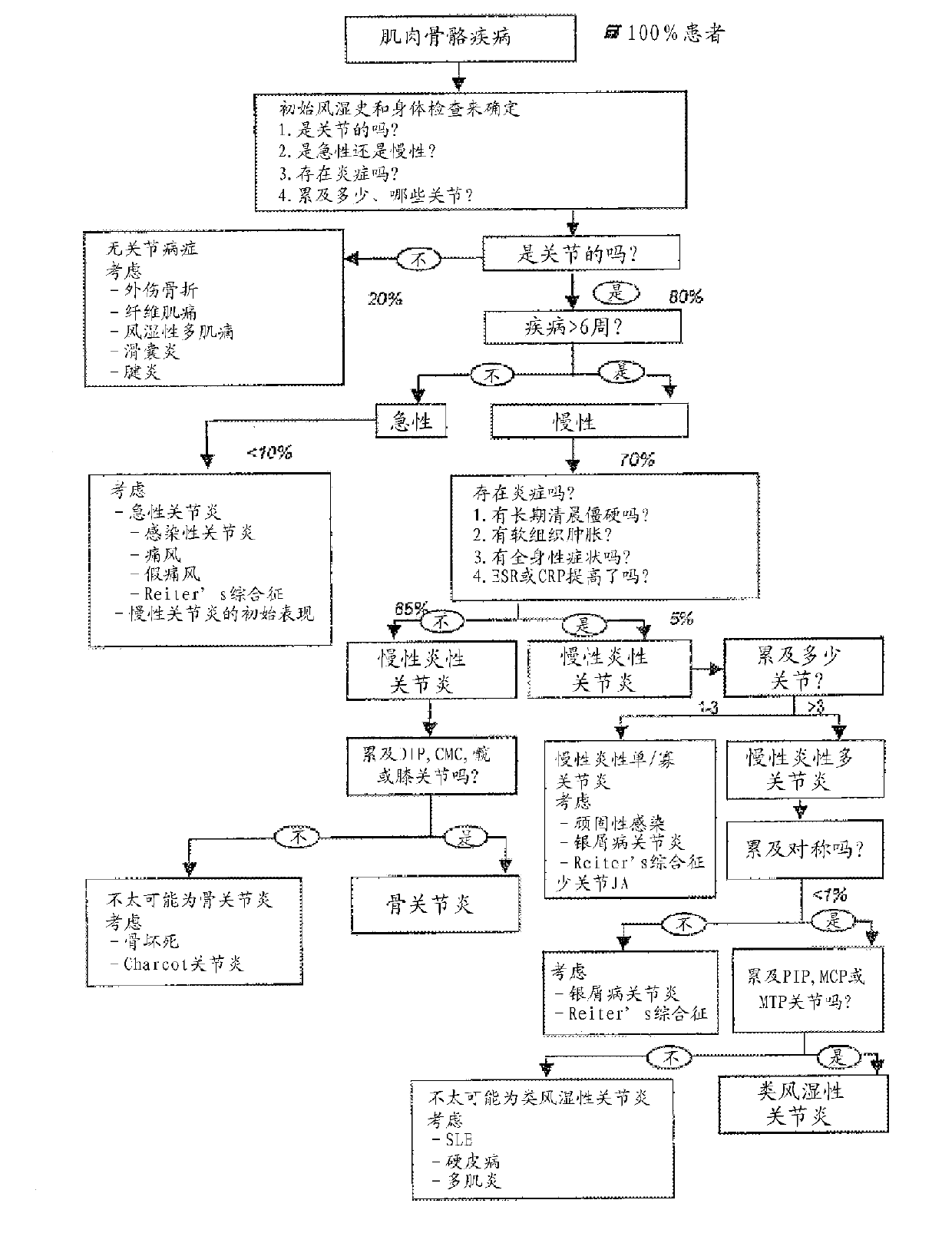
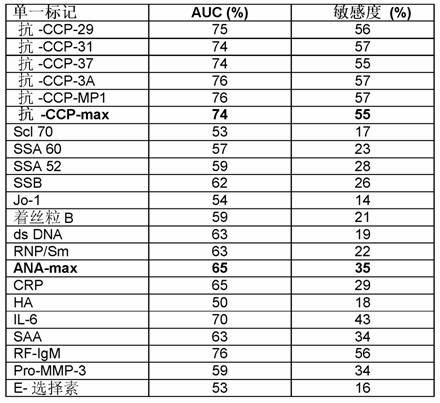
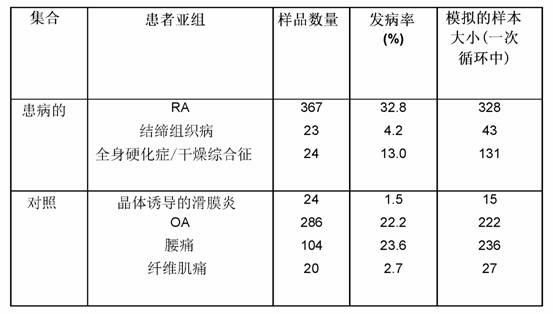
Trigger assay for differentiating between rheumatic and non-rheumatic disorders
A rheumatic and rheumatoid technology, applied in the field of trigger measurement for distinguishing rheumatic and non-rheumatic diseases, which can solve problems such as wrong treatment of diseases and patient deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0278] Example 1: Immune multi-parameter chip technology IMPACT-general operation
[0279] A black polystyrene chip with a surface area of approximately 2.5 x 6 mm was completely coated with a layer of streptavidin. On this streptavidin surface we applied the same reagent dots in rows (approximately 20 dots per reagent per row) using the ink-jet technique. Each spot is approximately 150 μm in diameter and contains a biotinylated binding reagent capable of specifically binding to an analyte (eg, antigen or antibody) in the sample.
[0280] Each sample was diluted with sample dilution buffer and 40 μl of the diluted sample was applied and incubated per chip. Assays were performed on automated pre-prototype instruments.
[0281] Detection Specific Reagents
[0282] Sample Dilution Buffer: 50mM Tris, 30mM MES, 50mM NaCl, 5mM EDTA, 0.5% Casein, 0.1% Detergent (Polydocanol), 0.2% Preservative (Oxypyrion and Methylisothiazole Hydrochloride (MIT))
[0283] Wash buffer: 10mM Tris...
Embodiment 2
[0286] Embodiment 2: Composition of multi-parameter array
[0287] After the multiplex array kit was produced, the sample set was assayed for the following analytes:
[0288] Multi-1: RF-IgM, CRP, SAA
[0289] Multiplex-2: 5 different anti-CCP-autoantibodies: 5 different peptides (cyclic citrullinated peptides) are coated onto the surface
[0290] Multiplex-3: Anti-centromere B peptide, anti-SSA52, anti-Jo-1, anti-SSA60, anti-SSB, anti-Scl70, anti-RNP / Sm, anti-dsDNA
[0291] Multiplex 4: IL-6, prohyaluronan (HA)-MMP-3, E-selectin
Embodiment 3
[0292] Example 3: Study Population
[0293] patient
[0294] Patients were enrolled in a prospective longitudinal study in six European trial centers according to a harmonized standard operating procedure (SOP). Samples from patients with osteoarthritis (OA) were collected at Bristol (UK), samples from patients with autoimmune disease and controls were collected at Chur (CH), and samples from patients with RA were collected at five centres. In this experiment, only the samples at the time of enrollment were used. Inclusion criteria for the RA cohort were the presence of a minimum of four ACR criteria plus a retrospective diagnosis after 2 years of follow-up (Hochberg, M.C. et al., Arthritis Rheum. 35 (1992) 498-502). All RA, autoimmune, and control patients had established disease with a positive diagnosis verified by each center. The trial was approved by the ethics committee of each participating center.
[0295] case report form
[0296] A standard case repor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com