Polyasparagine-L-arginine and preparation method and medical application thereof
A technology of polyaspartic acid and arginine is applied in the application of polyaspartic acid-L-arginine as an antithrombotic agent, in the field of polyaspartic acid-L-arginine, which can solve the Amino acid blends don't have issues like exact doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
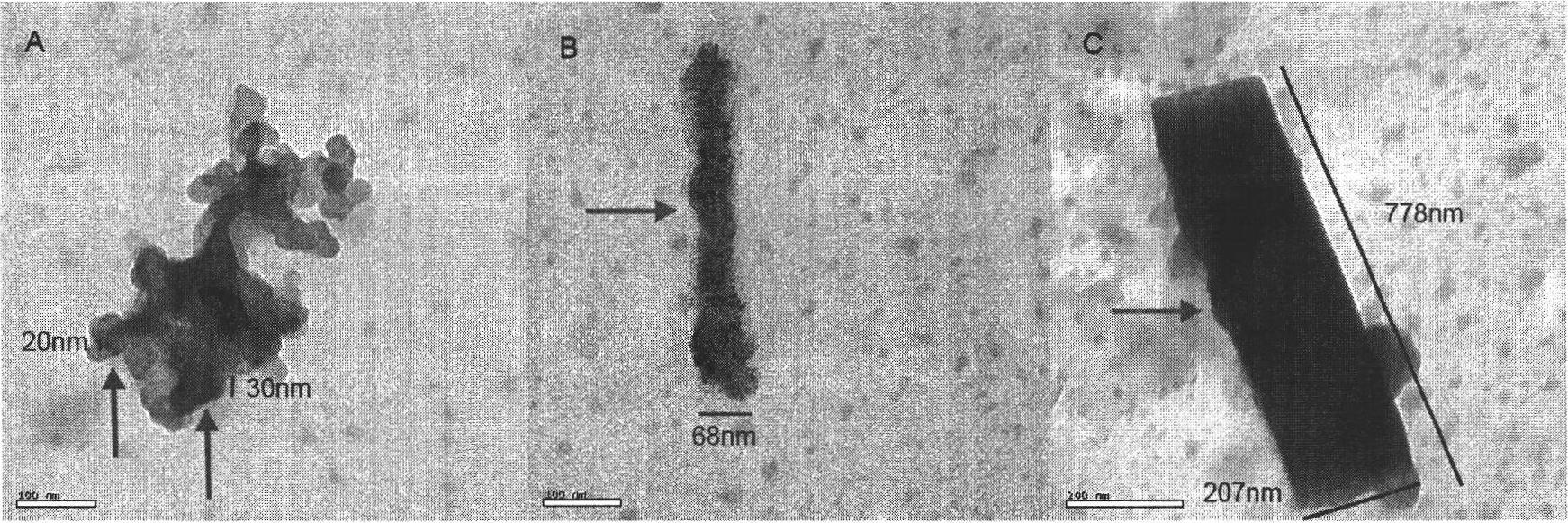
Image
Examples
Embodiment 1
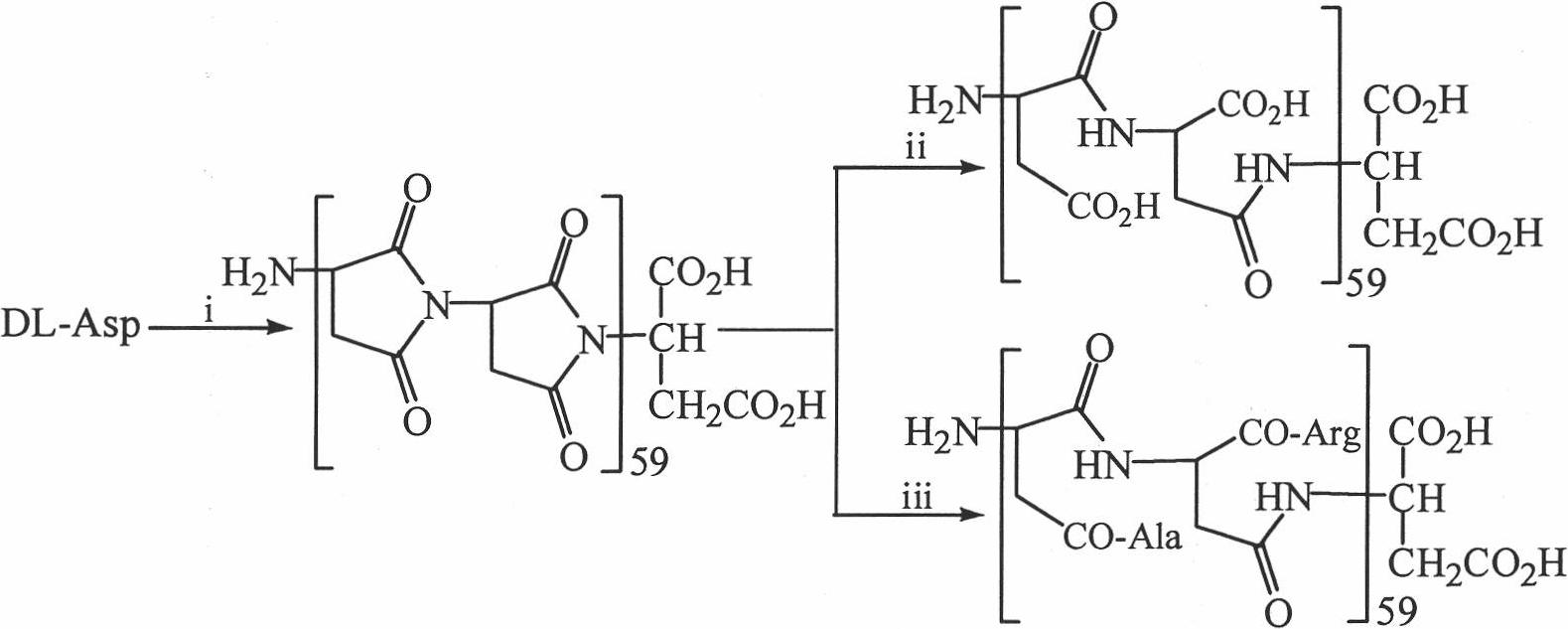
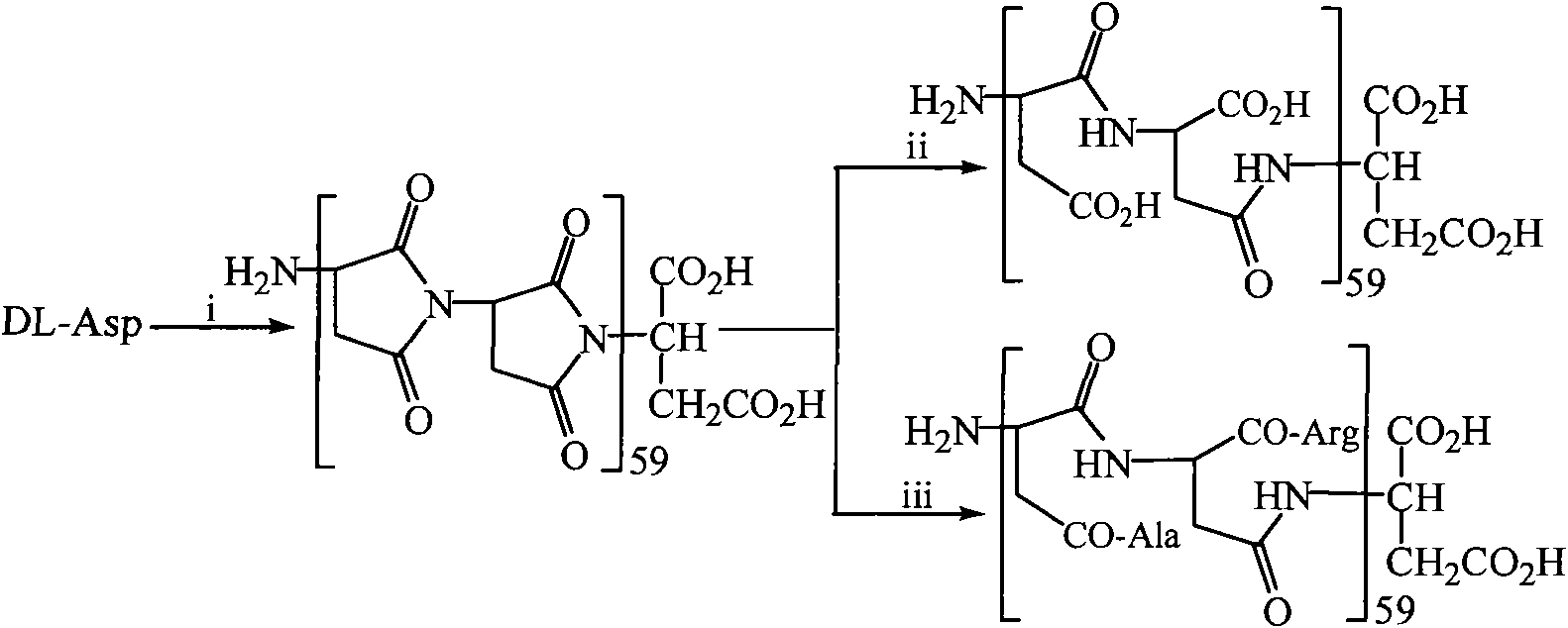
[0030] Embodiment 1 Heating and decompression method prepares the polysuccinimide that chain length is 59
[0031] 5 g of finely ground DL-aspartic acid, 2 ml of phosphoric acid (85%) and 2 ml of distilled water were mixed thoroughly in a 250 ml round bottom flask. The reaction mixture was reacted under reduced pressure in an air bath at 180°C for 2.5 hours, and then 20ml of DMF was added to it while it was still hot. After the solution became clear, it was dropped into 100ml of distilled water. The precipitate was collected, washed neutral with distilled water, and dried to yield 3.4 g (93.4%) of the title compound. Elemental analysis (C 4 h 3 NO 2 ) n : C, 46.76; H, 3.35; N, 13.64.
Embodiment 2
[0032] Embodiment 2 Azeotropic water removal method prepares polysuccinimide with a chain length of 59
[0033] A suspension of 50 g of finely ground DL-aspartic acid and 500 ml of tetralin (chemical alcohol) was refluxed for 100 hours, and the generated water was removed through a water separator. After the reaction mixture was cooled to room temperature, it was filtered, and the filter residue was washed with ether first, and then washed with saturated NaHCO 3 Wash with aqueous solution (3 x 100ml). The filter cake was washed repeatedly with water and dilute hydrochloric acid (1%), and finally washed repeatedly with distilled water until AgNO 3 Cl cannot be detected - . The filter cake was dried to yield 16 g (44%) of the title compound. Elemental analysis (C 4 h 3 NO 2 ) n : C, 45.24; H, 3.85; N, 13.30.
Embodiment 3
[0034] Embodiment 3 melting method prepares the polysuccinimide that chain length is 59
[0035] 30g of finely ground DL-aspartic acid was evenly spread on the bottom of a vessel with a diameter of 30cm, heated at 200°C for 3 hours, and the reaction product was orange-red. After the reactant was cooled, the saturated NaHCO 3After triturating with aqueous solution (3×100ml), the obtained solid was repeatedly washed with distilled water, centrifuged, and the precipitate was dried to obtain 5 g (17%) of the title compound. Elemental analysis: (C 4 h 3 NO 2 ) n : C, 46.63; H, 3.33; N, 13.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com