Lyophilized potassium sodium dehydroandroan drographolide succinate multi-vesicular liposome (MVL) powder and preparation method thereof
A multivesicular liposome, Yanhuning technology, applied in the field of liposome and its preparation, can solve the problems such as leakage of encapsulated substances, achieve reduced swelling rate, good physical stability and antioxidant capacity, and less adverse reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Precisely weigh 60 mg of hydrogenated soybean lecithin, 30 mg of cholesterol, and 1 mg of glyceryl trilaurate and dissolve them in 2.4 ml of chloroform as the lipid phase;
[0026] 2. Preparation of Rosa Rosa extract: add 500 mg of water to 100 mg of washed Rosa Rosa fruit, ultrasonically break for 1 min, the ultrasonic frequency is 20 kHz, and centrifuge for 3 min at 10,000 rpm, take the supernatant to obtain Rosa Rosa Fruit Extraction solution;
[0027] 3. Precisely weigh 80 mg of Yanhuning and dissolve it in water, add the extract of Rosa roxburghii obtained in step 2 as the inner water phase, so that the final concentration of Yanhuning is 40 mg / ml;
[0028] 4. Precisely weigh 99mg of glucose and dissolve it in 11ml of water, as the external water phase, the concentration of glucose is 9mg / ml;
[0029] 5. Slowly add 2ml of the inner water phase to the upper layer of 2.4ml of the lipid phase, and use a high-speed shear homogenizer to act on it at a speed of 10,00...
Embodiment 2
[0035] 1. Precisely weigh 60 mg of hydrogenated soybean lecithin, 30 mg of cholesterol, and 1 mg of glyceryl trilaurate and dissolve them in 2.4 ml of chloroform as the lipid phase;
[0036] 2. Precisely weigh 80mg of Yanhuning and dissolve it in water as the inner water phase, so that the final concentration of Yanhuning is 40mg / ml;
[0037] 3. Precisely weigh 99mg of glucose and dissolve it in 11ml of water, as the external water phase, the concentration of glucose is 9mg / ml;
[0038] 4. Slowly add 2ml of the inner water phase to the upper layer of 2.4ml of the lipid phase, and use a high-speed shear homogenizer to act on it at a speed of 10,000rpm for 10min to obtain a water-in-oil emulsion;
[0039] 5. Add the above-mentioned emulsion to 11ml of the external water phase, and use a high-speed shear homogenizer to act on it at a speed of 10,000rpm for 10min to obtain a water-in-oil-in-water type double emulsion;
[0040] 6. Add the double emulsion into a 500ml Erlenmeyer fl...
Embodiment 3
[0044] Animal experiment
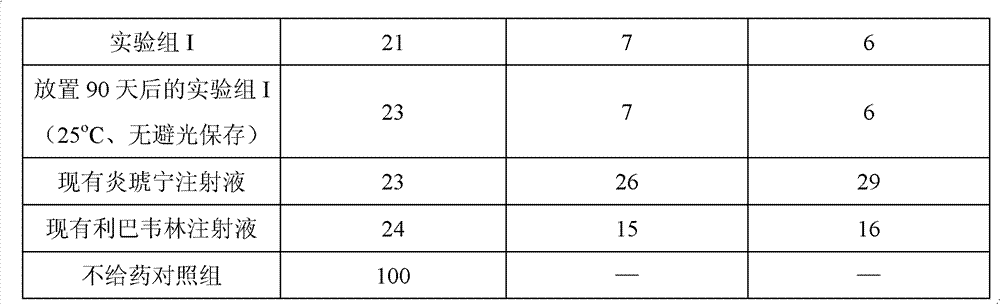
[0045] 1. Experimental animals: healthy Kunming mice, weighing 18-20 grams, were randomly divided into 5 groups, 100 in each group, half male and half male.
[0046] 2. Establishment of mouse experimental pneumonia model: intranasal infection of mice with A1 influenza virus, 10LD 50 The amount of virus was administered by tail vein injection 2 hours later.
[0047]3. Administration method: the positive drug control group selects the existing ribavirin injection and the existing Yanhuning injection, wherein: the existing ribavirin injection, the existing Yanhuning injection, the experimental group I, placed After 90 days, the dosage of the active ingredients in the experimental group I (25°C, no dark storage) was 50 mg / kg, once a day, administered for 6 days, observed for 2 weeks, and recorded the number of pneumonia deaths and the incidence of adverse reactions. And set no drug group. The results are shown in Table 1.
[0048]
[0049]
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com