Composition for down-regulating pro-inflammatory markers
A technology of composition and synergistic composition, applied in the direction of drug combination, preparation for in vivo test, medical raw material derived from angiosperm subphylum, etc., can solve problems such as danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Biological Activity Evaluation
[0062] Acute safety studies:
[0063] Acute oral toxicity studies were performed in mice in accordance with OECD Guideline No. 423 [Organization for Economic Co-operation and Development. OECD guidelines on chemical testing. Guideline 423, acute oral toxicity-acute toxicity class method, adopted, March 22, 1996 (Organization for Economic Cooperation and Development. OECD guidelines for testing of chemicals. Guideline 423, acute oral toxicity-acute toxic class method, adopted, March 22, 1996)] carried out. Animals were observed individually during the first 24 hours periodically, with special attention during the first 4 hours, and once daily thereafter, for a total of 14 days. A single oral (p.o.) dose of the composition administered orally to groups of female mice at 2000 mg / kg did not show any changes in the general general behavior of these test animals. Oral (p.o.) single doses of 5000 mg / kg were also evaluated. At th...
Embodiment 2
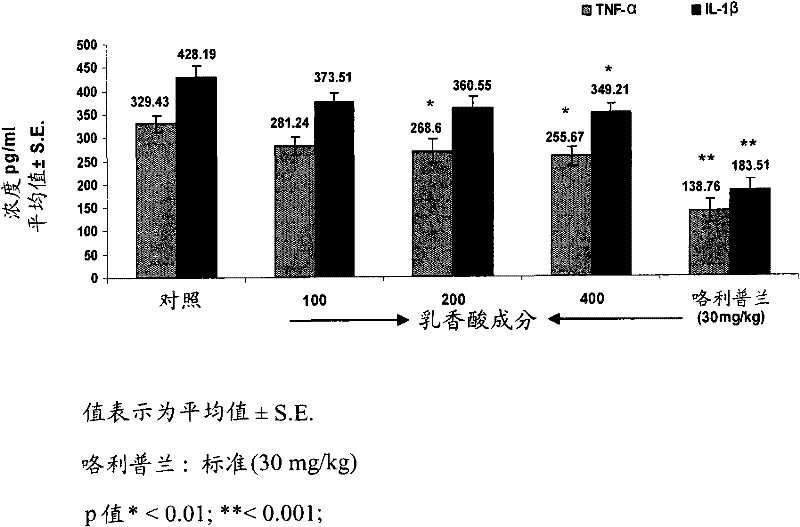
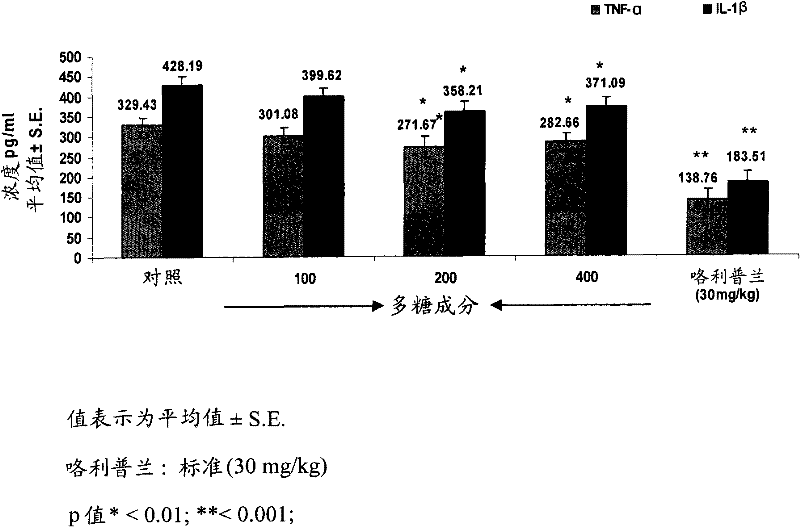
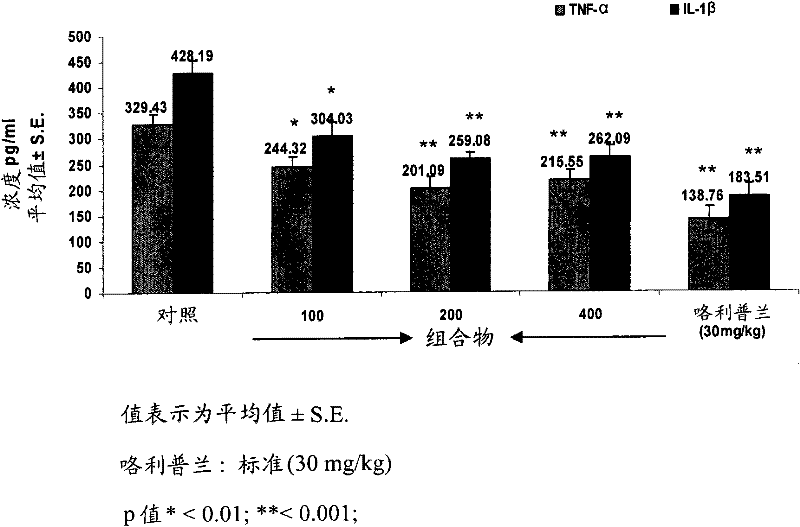
[0081] Example 2: Determination of Intracellular TNF-α, IL-1β and Nitric Oxide (NO) in vivo in Plasma from Treated Mice
[0082] BALB / c male mice aged 6-8 weeks were maintained at 22±2°C under a 12 / 12h light-dark cycle. Mice received 100, 200, 400 mg / kg of different test materials (w / v), i.e., boswellic acid components, polysaccharide components and compositions of the present invention, for 6 days, followed by oral treatment according to Brieva et al., 2001.[ Brieva A, Guerrero A, Alonso-Lebrero J Land Pivel JP. 2001. Inmunoferon, a glycoconjugate of natural origin, inhibits LPS-induced TNF-a production and inflammatory responses. International Immunopharmacology 1.1979-1987] Intravenous injection of 1 mg / kg The LPS. Six mice were used per group and experiments were performed in triplicate. Ninety minutes after LPS injection, TNF-α, IL-1β and nitric oxide production were assessed by commercial ELISA kits (R&D Systems) in mice treated from each test group. Rolipram at 30 mg...
Embodiment 3
[0084] Example 3: Adjuvant-induced developing inflammatory arthritis:
[0085] Six 12-14-week-old Wistar rats weighing 140-160 g per group were used in this study. All animals were maintained in plastic cages at 22±2°C under a 12h light / dark cycle and had free access to pelleted food and water. During the experiment, the test material was orally administered once a day. In all experiments, the control group was fed (maintained) (vehicle administered) while the other groups received the standard drug acetylsalicylic acid (ASA), administered once daily, for comparison and authenticity / authenticity of the test. The entire study was performed with permission from the Institutional AnimalEthics Committee and all animals used in the experimental work received humane care. The mean and standard error of the mean (S.E.) were calculated for each group and the results expressed as percent inhibition compared to the control group. Significance was determined statistically by applying ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com