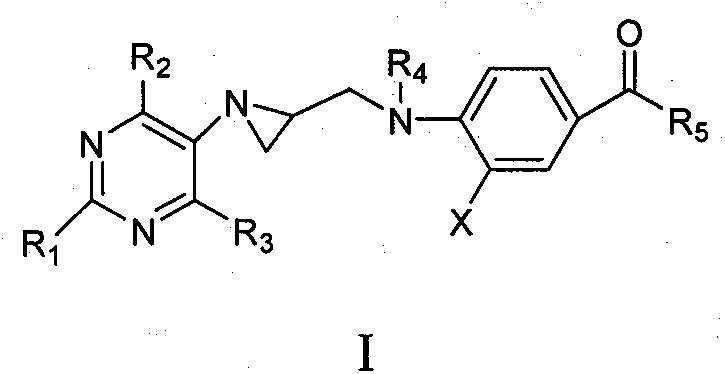
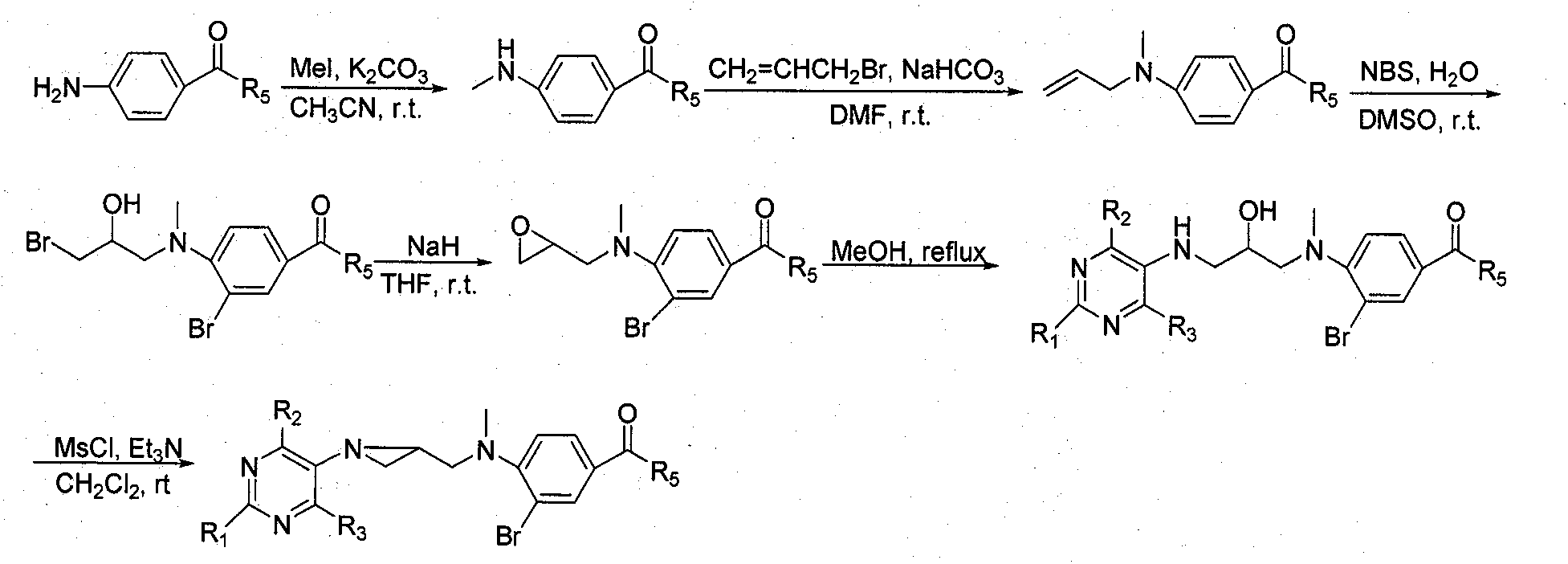
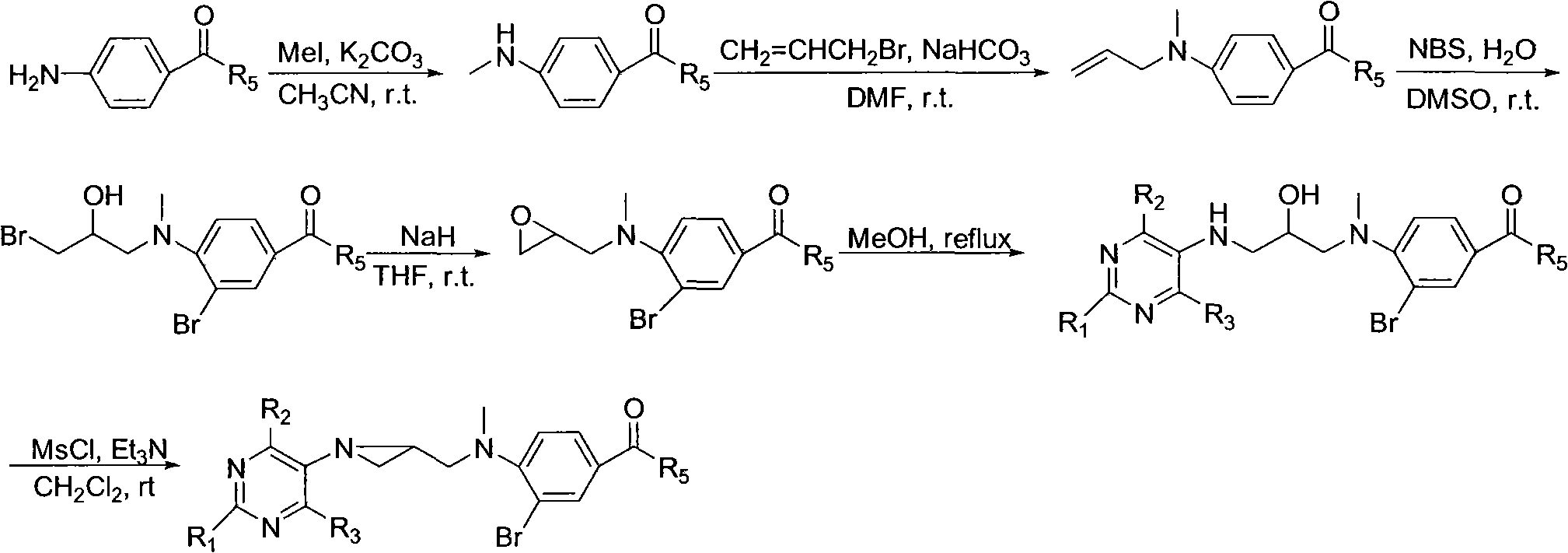
Preparation and application of tetrahydrofolic acid ring-opened analogs with aziridine structure
A kind of technology of aziridine and tetrahydrofolate, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Compound 1: Ethyl 4-methylaminobenzoate
[0014]
[0015] Ethyl 4-aminobenzoate (1.65g, 10mmol) was dissolved in 15mL of dry acetonitrile, anhydrous K was added 2 CO 3 (0.76g, 5.5mmol), stirred for 15 minutes and added CH 3 I (0.69mL, 11mmol), stirred at room temperature for 48h. Inorganic salts were removed by filtration, and the filtrate was concentrated under reduced pressure and then purified by silica gel column chromatography. The eluent was petroleum ether: ethyl acetate (9:1), and 0.77 g of the title compound was obtained as a white solid. The yield was 62%, m.p.61~ 62°C.
[0016] Compound 2: Diethyl N-(4-methylamino)benzoyl-L-glutamate
[0017]
[0018] According to the preparation method of compound 1, 1.05 g of the title compound was obtained as a white solid, with a yield of 64%, m.p.88-89°C.
[0019] Compound 3: Ethyl 4-(N-methyl-N-allyl)aminobenzoate
[0020]
[0021] Dissolve ethyl 4-methylaminobenzoate (1.79 g, 10 mmol) in anhydrous DMF (1...
Embodiment 2
[0120] Example 2 Evaluation of the biological activity of methionine synthase and dihydrofolate reductase on the synthesized compound
[0121] 1. Determination of inhibitory activity against methionine synthase (MS)
[0122] 1.1 Experimental principle:
[0123] Tetrahydrofolate, one of the products of the enzymatic reaction, can quantitatively react with formic acid solution under strong acidic and heating conditions, and derivatize to generate CH+=H 4 Folate has strong absorption at 350nm. The reaction substrate methyltetrahydrofolate and other compounds in the reaction system do not react with formic acid, and the absorption at 350nm is very small. Therefore, the stronger the inhibitory effect of the test compound on methionine synthase, the less the amount of tetrahydrofolate produced after the enzymatic reaction, and the weaker the absorption at 350nm.
[0124] 1.2 Experimental method:
[0125] Take HL-60 cells in the logarithmic growth phase, and use 1×10 6 The numbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com