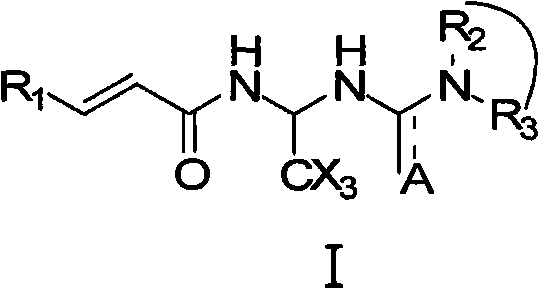
Acrylamide compounds and medicinal uses thereof
A technology of acrylamide and compounds, which is applied in the field of acrylamide compounds and their medical applications, and can solve the problems of low selectivity and targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
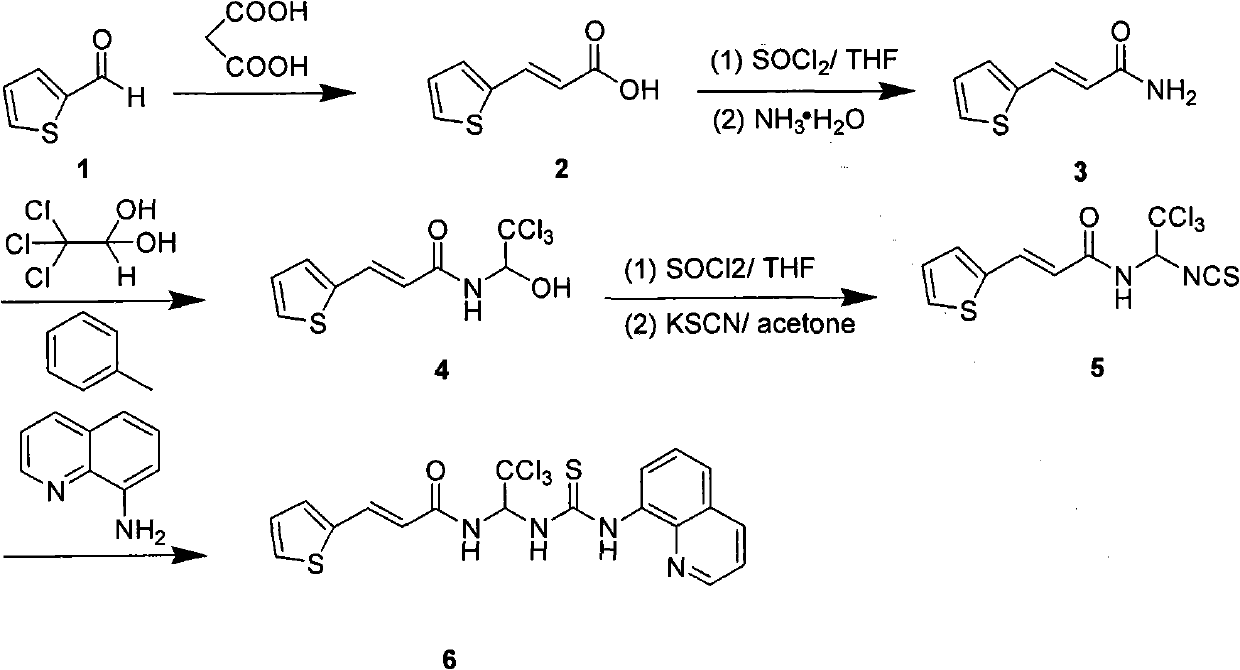
[0072] Example 1 (2E)-3-(2-Thienyl)-N-[1-(8-quinolylamino)thioformylamino-2,2,2-trichloroethyl]-2-acrylamide
[0073]
[0074]Dissolve 1.12g of 2-thiophenecarbaldehyde and 3.12g of malonic acid in 15ml of pyridine, add a catalytic amount of piperidine, react at 80°C for 6 hours, pour the reaction solution into 20ml of water, adjust the pH to 12 with 10% NaOH aqueous solution, and extract with ethyl acetate. The aqueous layer was adjusted to pH 3 with 2N hydrochloric acid, and a large amount of yellow solid (2E)-3-(2-thienyl)acrylic acid was precipitated, which was filtered and washed with water to obtain 1.12 g. Dissolve (2E)-3-(2-thienyl)acrylic acid in 10ml of anhydrous dichloromethane, add a catalytic amount of DMF, add 1.5ml of oxalyl chloride dropwise in an ice-water bath, warm up to room temperature and stir for 2 hours after dropping, the reaction solution Drop into 10ml of concentrated ammonia water at 0°C, stir for 30min, separate the dichloromethane layer and eva...
Embodiment 2
[0075] Example 2 (2E)-3-(3-Thienyl)-N-[1-(8-quinolylamino)thioformylamino-2,2,2-trichloroethyl]-2-acrylamide
[0076]
[0077] Using the method of Example 1, the 2-thiophenecarbaldehyde was changed to 3-thiophenecarbaldehyde to obtain 0.60 g of a yellow solid. 1 H-NMR (400MHz, DMSO-d 6 )δ6.67-6.71(d, 1H); δ7.35-7.36(d, 1H); δ7.54-7.72(m, 6H); δ7.87-7.88(d, 1H); δ8.42-8.44 (dd, 1H); δ 8.96-9.03 (m, 3H); δ 9.54-9.56 (d, 1H); δ 11.05 (s, 1H). MS (TOF) 487.0 (M+).
Embodiment 3
[0078] Example 3 (2E)-3-(2-Thienyl)-N-[1-(4-methylphenylamino)thioformylamino-2,2,2-trichloroethyl]-2-acrylamide
[0079]
[0080] Using the method of Example 1, the 8-aminoquinoline was changed to 4-methylaniline to obtain 60 mg of yellow solid. 1 H-NMR (400MHz, DMSO-d 6 )δ2.28(s, 3H); δ6.47-6.51(d, 1H); δ7.13-7.18(m, 3H); δ7.36-7.45(m, 4H); δ7.66-7.72(m , 2H); δ8.03(, 1H); δ8.96-8.98(d, 1H); δ10.21(s, 1H). MS (TOF) 450.0 (M+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com