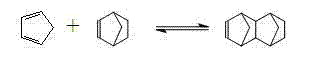
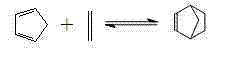
Method for preparing norbornene in loop reactor
A loop reactor and norbornene technology, applied in the direction of addition of unsaturated hydrocarbons to hydrocarbons, etc., can solve problems such as harsh operating conditions, low ethylene reactivity, and difficult realization, and achieve fast heat transfer and high raw material selection The effect of improving sex and monomer conversion rate and safety factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The loop reactor used in this example has a length of 30 cm, an inner diameter of 2 cm, and a static mixer at the inlet. Dicyclopentadiene is partially or completely decomposed into cyclopentadiene by heating and pre-decomposing in a heat exchanger before entering the loop reactor, and the process conditions are 240°C and 5MPa. When ethylene enters the loop reactor, the temperature is 180°C and the absolute pressure is 5MPa. The pre-decomposition products of ethylene and dicyclopentadiene are respectively fed through metering pumps, and are fully mixed through static mixers when entering the loop reactor. Wherein, the feed flow rate of ethylene is 6.24mL / s, the feed flow rate of the pre-decomposition product of dicyclopentadiene is 1.41mL / s, the ethylene added to the loop reactor and the dicyclopentadiene before heating pre-decomposition The molar ratio of alkenes is 1. The temperature of the loop reactor is 180°C, the absolute pressure at the outlet is 5MPa; the mate...
Embodiment 2
[0050] The loop reactor used in this example has a length of 30 cm, an inner diameter of 2 cm, and a static mixer at the inlet. Dicyclopentadiene is partially or completely decomposed into cyclopentadiene by heating and pre-decomposing in a heat exchanger before entering the loop reactor, and the process conditions are 120°C and 8MPa. When ethylene enters the loop reactor, the temperature is 220°C and the absolute pressure is 10MPa. The pre-decomposition products of ethylene and dicyclopentadiene are respectively fed through metering pumps, and are fully mixed through static mixers when entering the loop reactor. Wherein, the feed flow rate of ethylene is 4.64mL / s, the feed flow rate of the pre-decomposition product of dicyclopentadiene is 0.75mL / s, the ethylene added to the loop reactor and the dicyclopentadiene before heating pre-decomposition The molar ratio of alkenes is 2. The temperature of the loop reactor is 220°C, the absolute pressure at the outlet is 10MPa; the ma...
Embodiment 3
[0052] The loop reactor used in this example has a length of 30 cm, an inner diameter of 2 cm, and a static mixer at the inlet. Dicyclopentadiene is partially or completely decomposed into cyclopentadiene by heating and pre-decomposing in a heat exchanger before entering the loop reactor, and the process conditions are 150°C and 13MPa. The temperature of ethylene entering the loop reactor is 260°C and the absolute pressure is 15MPa. The pre-decomposition products of ethylene and dicyclopentadiene are respectively fed through metering pumps, and are fully mixed through static mixers when entering the loop reactor. Wherein, the feed flow rate of ethylene is 3.27mL / s, the feed flow rate of the pre-decomposition product of dicyclopentadiene is 0.11mL / s, the ethylene added to the loop reactor and the dicyclopentadiene before heating pre-decomposition The molar ratio of alkenes is 16. The temperature of the loop reactor is 260° C., and the absolute pressure at the outlet is 15 MPa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com