Methods of enhancing translocation of charged analytes through transmembrane protein pores
A technology for transmembrane protein pores and analytes, which is applied in the field of enhancing the translocation of charged analytes through transmembrane protein pores, and can solve problems such as laboriousness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
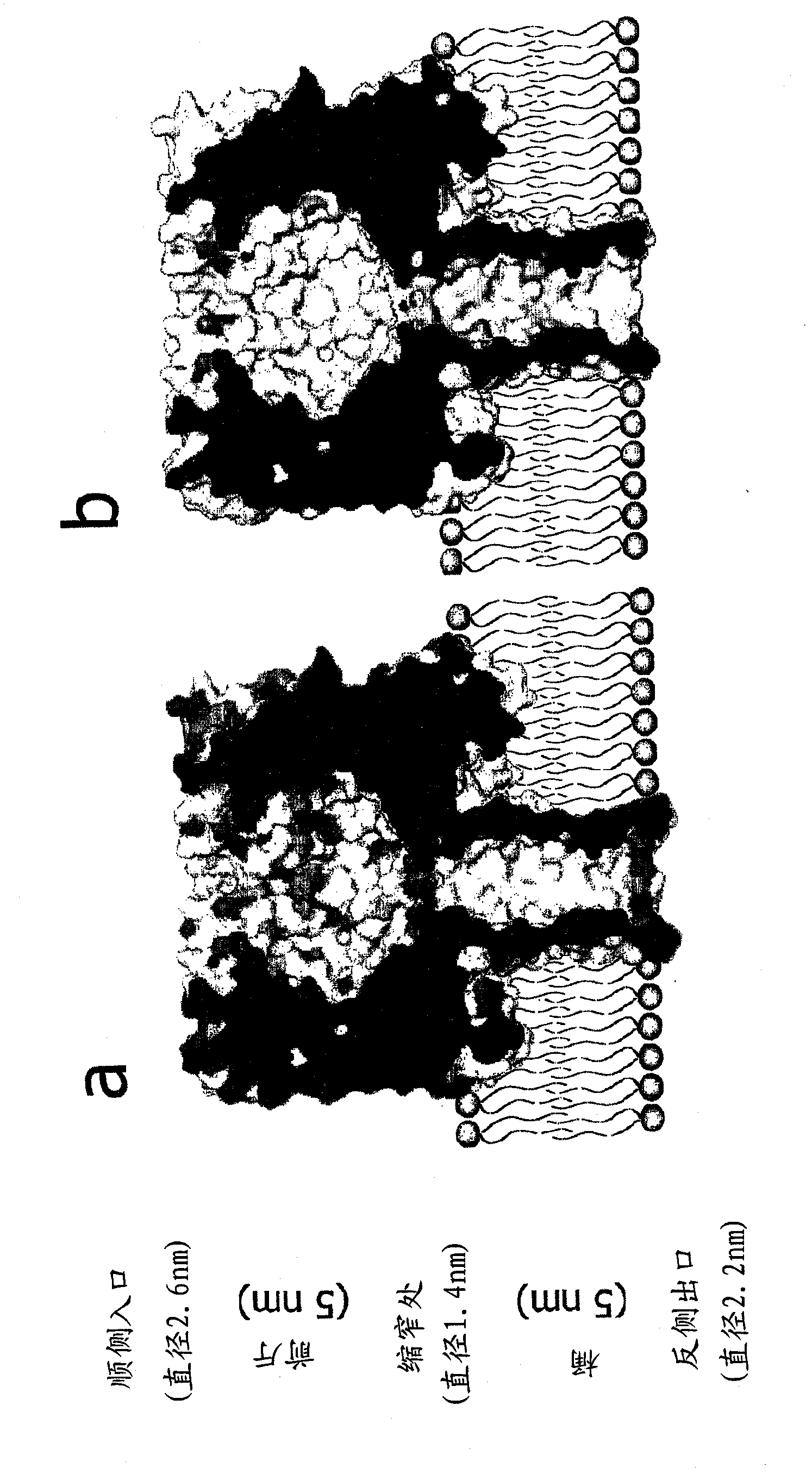
[0199] In this example, by increasing the internal positive charge of the α-hemolysin pore and altering its distribution, the inventors increased the capture rate of 92-nt ssDNA at +120mV by approximately 10-fold compared to wild-type pore and significantly decreased the voltage threshold for DNA translocation across the pore, e.g. for 1 event s -1 mM -1 reduced by 50mV. Furthermore, events where DNA entered the pore but was not translocated were virtually eliminated. These genetically engineered nanopores provide the basis for improved nucleic acid analysis and could facilitate ultrahigh-speed nanopore sequencing.
[0200] 1. Materials and Methods
[0201] 1.1 Protein preparation
[0202] α-HL was generated as described elsewhere (Cheley S, Braha O, Lu X, Conlan S, Bayley H: A functional protein pore with a "retro" transmembrane domain. Protein Sci. 1999, 8: 1257-1267). The sequence of one subunit of α-HL is shown in SEQ ID NO:2. Briefly, the protein in [ 35 [S]Met...
Embodiment 2
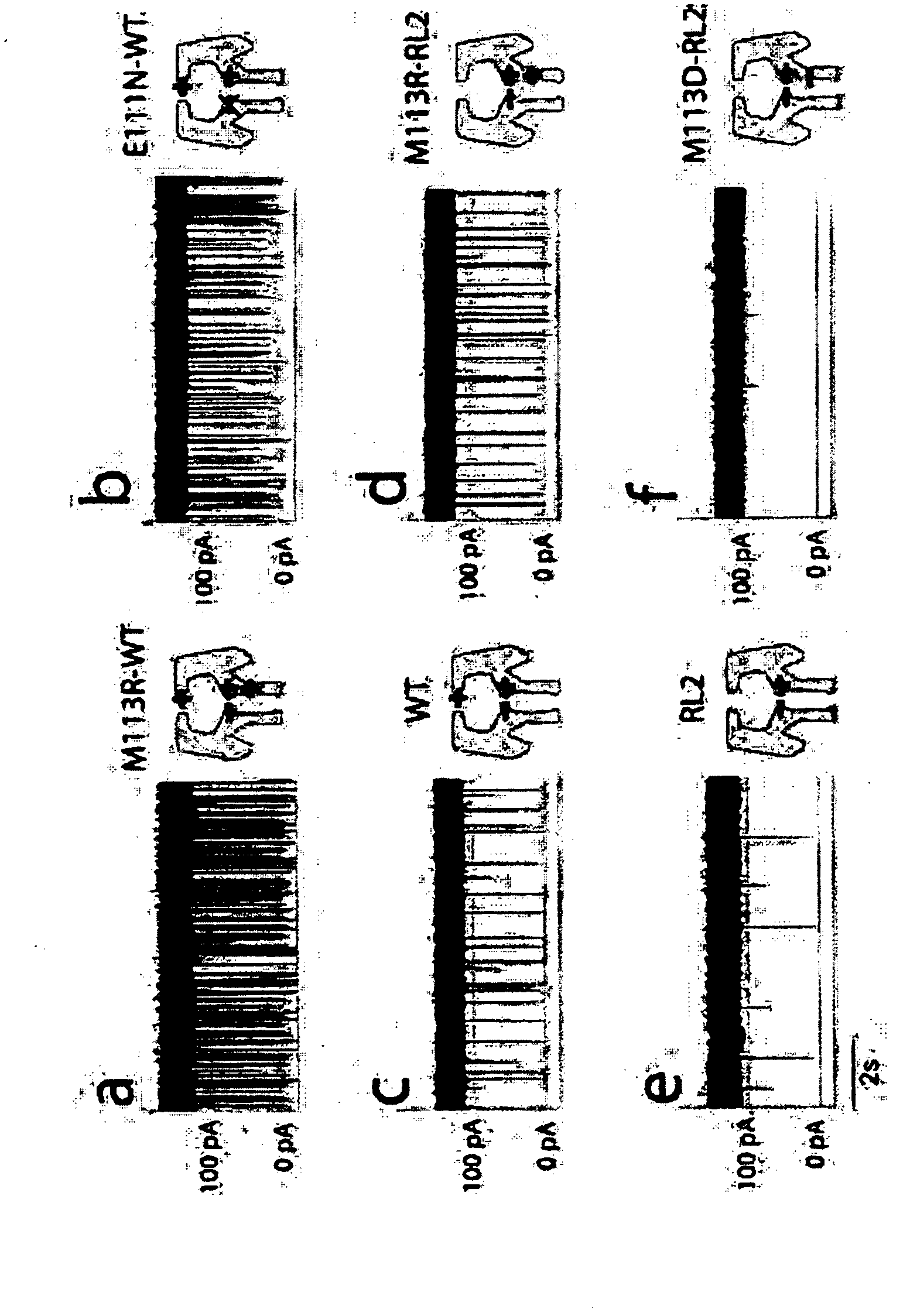
[0265] In this example, the inventors demonstrated that the translocation of DNA molecules through the α-HL bionanopore can be reduced by two orders of magnitude by introducing a positive charge within the cavity of the pore. Although the ionic currents during DNA translocation are almost completely suppressed, the inventors propose that these nanopores can be used to control the speed of DNA translocation, which in combination with emerging DNA analysis techniques could lead to the development of a robust platform for nucleic acid sequencing.
[0266] 1. Materials and Methods
[0267] The same materials and methods as used in Example 1 were used in this example.
[0268] 2. Results and Discussion
[0269] 2.1. Ion flow through the α-HL nanopore
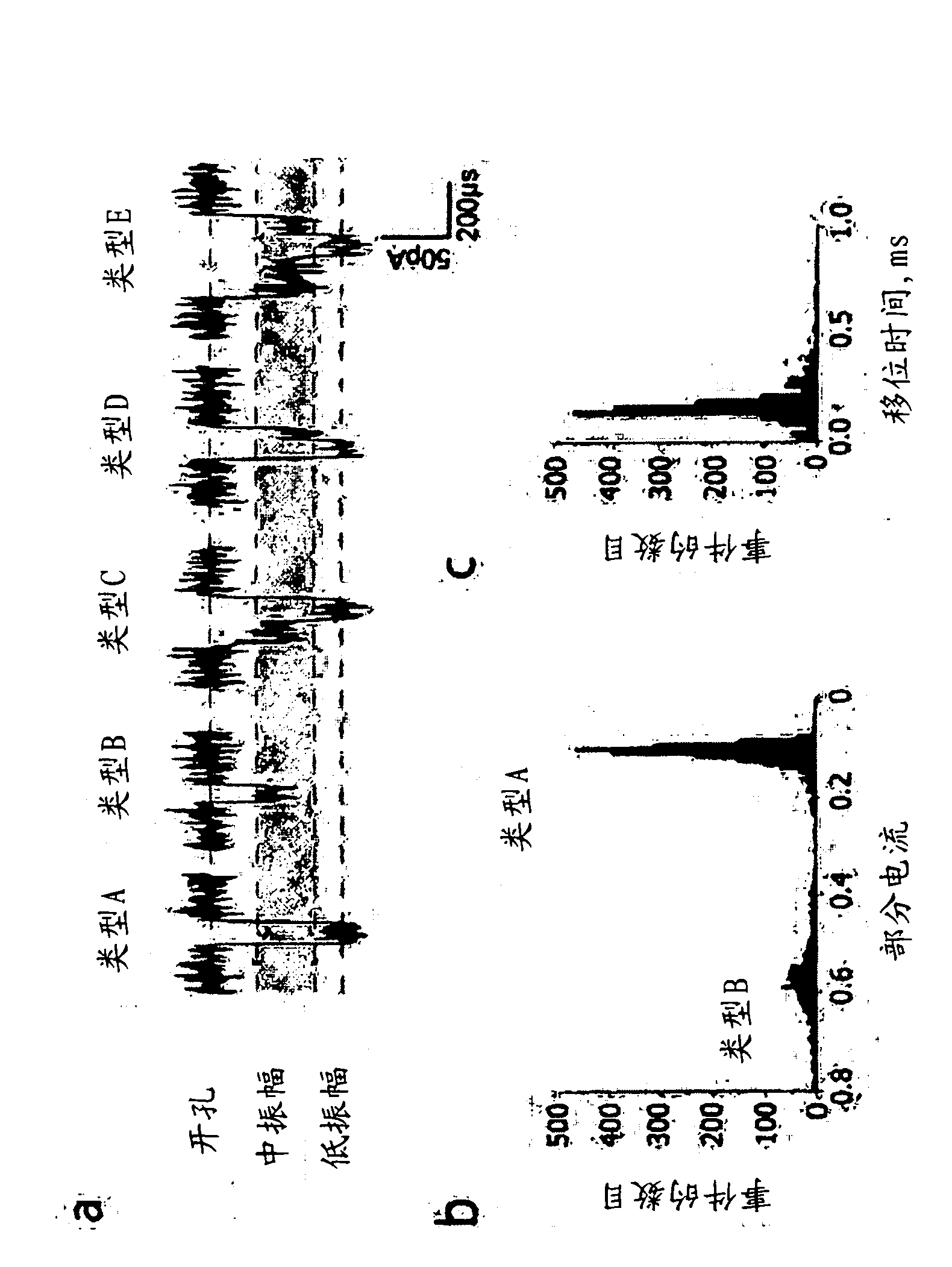
[0270] Individual channels were examined by single-channel current recordings on planar lipid bilayers in 1 M KCl, 25 mM Tris.HCl, pH 8.0, containing 100 [mu]M EDTA. At +120mV, all channels exhibited a steady open pore curre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com