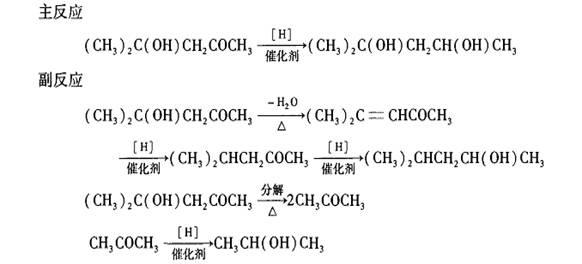
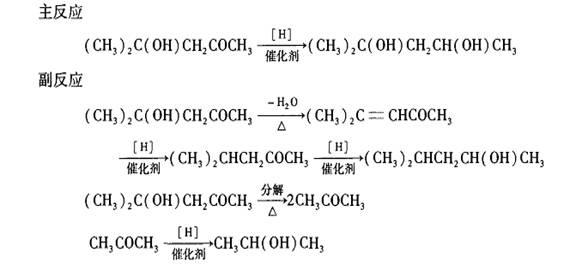
Process for synthesizing 2-methyl-2,4-pentendiol through hydrogenation reduction of diacetone alcohol
A technology of isohexanediol and diacetone alcohol, which is applied in the production process of isohexanediol, can solve problems such as difficult operation, difficult product separation, and high production cost, and achieve the effect of reducing unit consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] A specific example of implementation is given below, but the technical solution protected by the present invention is not limited to this example.
[0026] Using 2000 L of diacetone alcohol as raw material hydrogenation reduction synthesis of isohexanediol, its specific process steps are:
[0027] Step 1: Start the vacuum pump to evacuate the raw material metering tank. When the vacuum is greater than -0.06Mpa, close the pumping valve, open the feeding valve, and mix 2000 L of diacetone alcohol raw material and 200ppm of sodium bicarbonate and pump it in. Raw material metering tank.
[0028] Step 2: Open the vacuum valve on the reduction pot, pump the reduction pot to a vacuum of -0.1Mpa, then open the feed valve on the reduction pot, and add 2000 L diacetone alcohol raw material and 200ppm sodium bicarbonate from the metering tank To reduction pot, start stirrer to stir simultaneously, add the Raney nickel catalyst of 95Kg.
[0029] Step 3: Close the vacuum valve, op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com