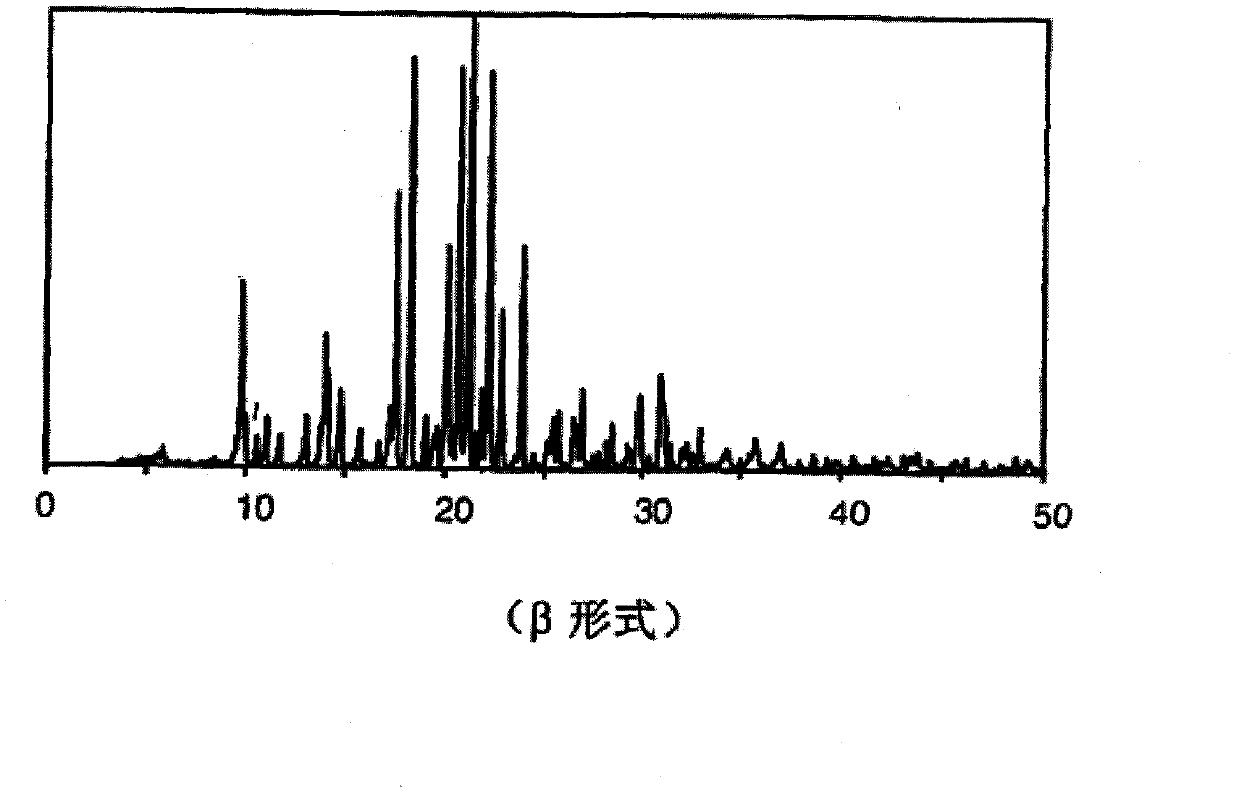
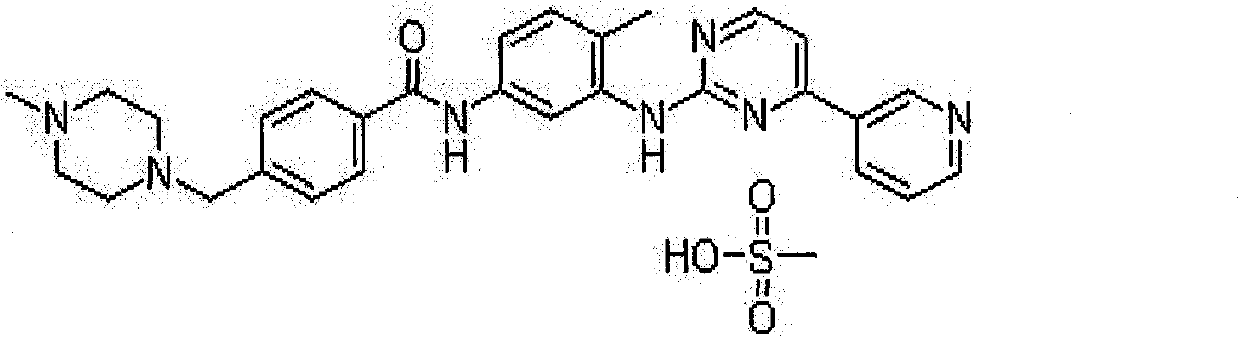
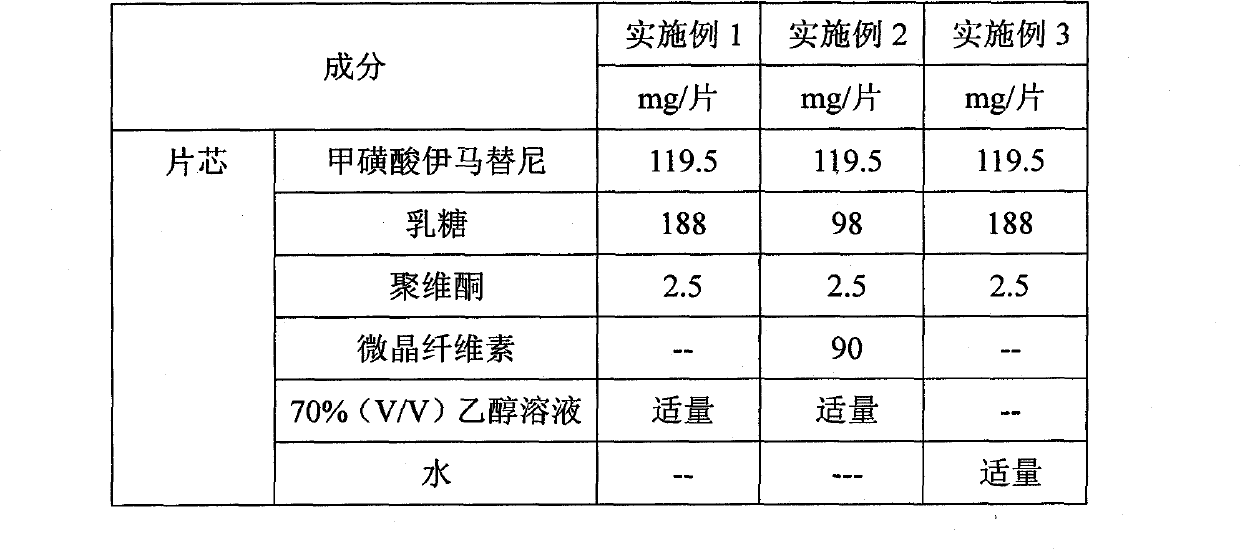
Preparation method of methylsulfonic acid imatinib tablet
A tablet-forming technology of imatinib mesylate, which is applied in the field of preparation of tablets containing imatinib mesylate, and can solve problems such as low dissolution and difficult dissolution of raw materials
Inactive Publication Date: 2012-02-15
ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
View PDF7 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, it was found that there was a problem of low dissolution
This may be due to the accumulation of insoluble excipients such as microcrystalline cellulose making it difficult to dissolve the raw material
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1-3
Embodiment 4-7
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a preparation method of methylsulfonic acid imatinib tablet. The invention takes organic solvent or organic solution with volume concentration bigger than 70% as pelletization solution, and the weight of water-insoluble filling agent in the tablet accounts for less than 20% of the total weight of the tablet. The invention has advantages of simple technical process, high yield and good dissolution of a product.
Description
technical field The invention relates to a preparation method of a tablet containing imatinib mesylate. Background technique Imatinib mesylate, developed by Novartis, is the world's first approved inhibitor of tumorigenesis-related signal transduction. Its chemical name is: 4-[(4-methyl-1-piperazine)methyl]-N-[4-methyl-3-[[4-(3-pyridine)-2-pyrimidine]amino]benzene base]-aniline methanesulfonate. Molecular formula: C 29 h 31 N 7 O·CH 4 SO 3 Molecular weight: 589.7 The simplified structure is: WO2009 / 042803 discloses the technology of producing imatinib mesylate tablets by dry granulation process, and the ratio of its raw material drug is 23-29%. EP1762230B1 protects the process of producing coated tablets, mainly dry granulation process, and the amount of API is between 25-80%. The production of imatinib mesylate tablets by dry granulation has certain defects in commercial production. Relatively speaking, the amount of dust generated in the dry granulation pr...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/20A61K31/506A61P35/00
Inventor 彭俊清代孔恩魏芳胡李斌李巧霞胡功允
Owner ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com