Method for preparing beta-naphthoxyacetic acid
A technology of naphthyloxyacetic acid and chloroacetic acid, which is applied in the field of preparation of β-naphthyloxyacetic acid, can solve the problems of expensive raw materials, unfavorable industrial production, and low yield, and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
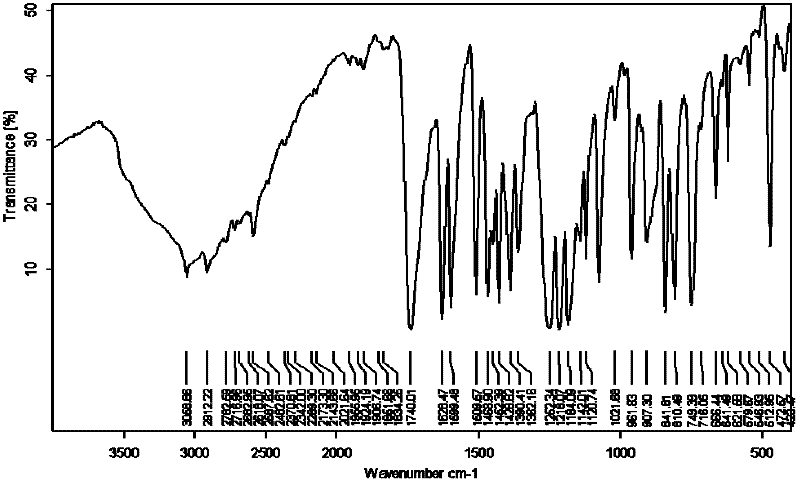
Image
Examples
Embodiment 1
[0017] In the first step, add 3g of sodium hydroxide and 7ml of water into the reactor and stir to dissolve the sodium hydroxide, then add 0.02mol β-naphthol, stir quickly to completely dissolve the β-naphthol, and react with β-naphthol to form sodium phenate.
[0018] In the second step, add 0.01mol chloroacetic acid and gradually raise the temperature to 80°C. At this time, all the solids are dissolved, and a dark brown solution is obtained. Continue to stir for 10 minutes, and a beige solid precipitates. Stop stirring and cool to room temperature, and add 30% HCl solution while stirring. Adjust the pH to 1; the color of the solid becomes darker, filter, wash with water, and recrystallize with benzene or 50% alcohol-water to obtain white crystals that are β-naphthyloxyacetic acid. Calculated by weighing, the yield is 57%.
Embodiment 2
[0020] In the first step, add 3g of sodium hydroxide and 7ml of water into the reactor and stir to dissolve the sodium hydroxide, then add 0.02mol β-naphthol, stir quickly to completely dissolve the β-naphthol, and react with β-naphthol to form sodium phenate.
[0021] In the second step, add 0.025mol chloroacetic acid and gradually raise the temperature to 80°C. At this time, all the solids are dissolved to obtain a dark brown solution. Continue to stir for 10 minutes and a beige solid will precipitate. Stop stirring and cool to room temperature. Add 30% HCl solution while stirring. Adjust the pH to 1; the color of the solid becomes darker, filter, wash with water, and recrystallize with benzene or 50% alcohol-water to obtain white crystals that are β-naphthyloxyacetic acid. Calculated by weighing, the yield is 65%.
Embodiment 3
[0023] In the first step, add 3g of sodium hydroxide and 7ml of water into the reactor and stir to dissolve the sodium hydroxide, then add 0.02mol β-naphthol, stir quickly to completely dissolve the β-naphthol, and react with β-naphthol to form sodium phenate.
[0024] In the second step, add 0.045mol chloroacetic acid and gradually heat up to 80°C. At this time, all the solids are dissolved to obtain a dark brown solution. Continue to stir for 10 minutes and a beige solid will precipitate. Stop stirring and cool to room temperature. Add 30% HCl solution while stirring. Adjust the pH to 1; the color of the solid becomes darker, filter, wash with water, and recrystallize with benzene or 50% alcohol-water to obtain white crystals that are β-naphthyloxyacetic acid. Calculated by weighing, the yield is 77%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com