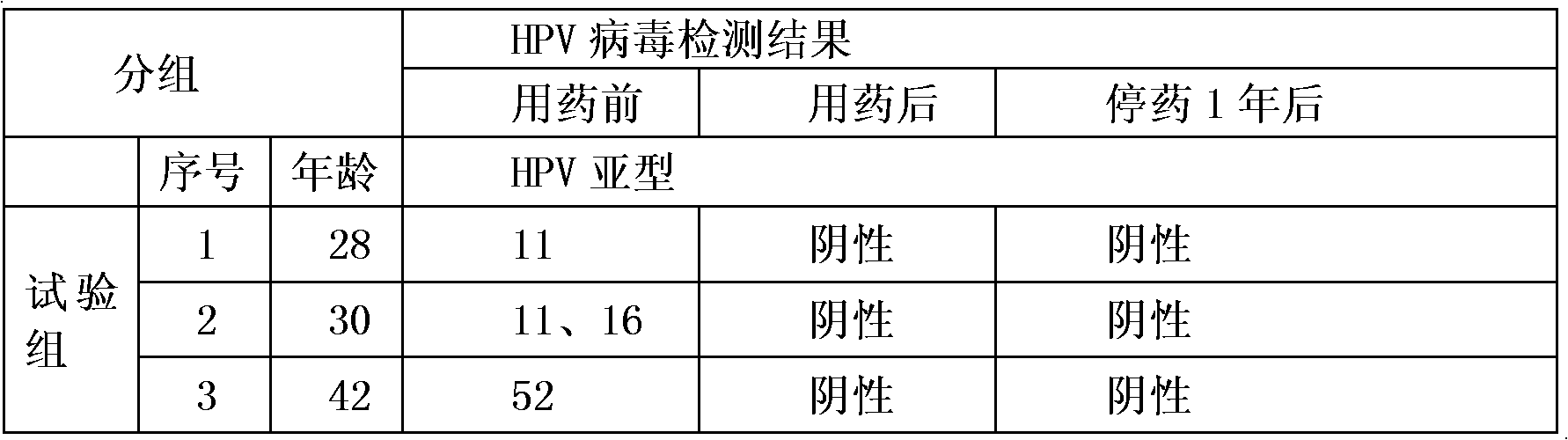
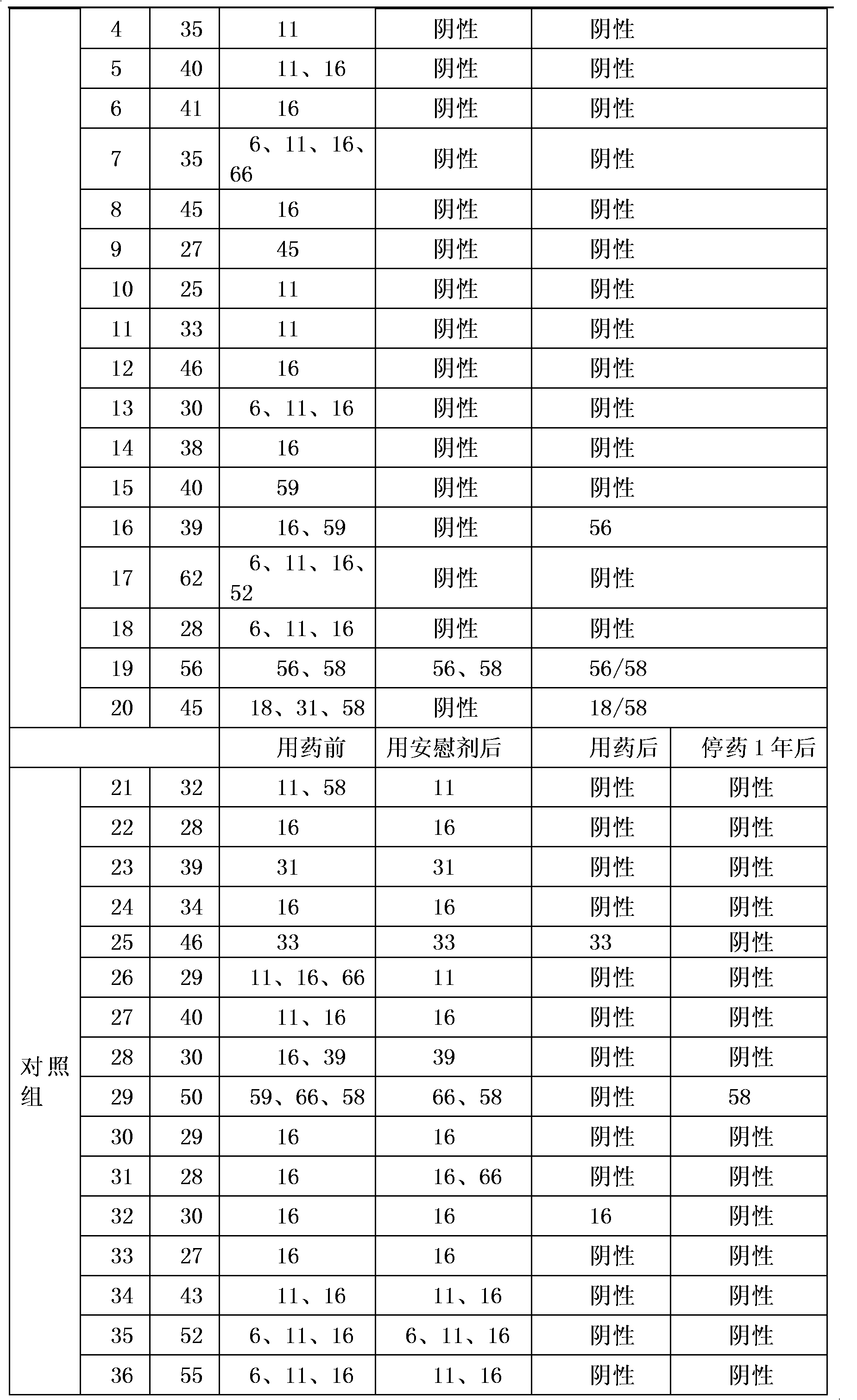
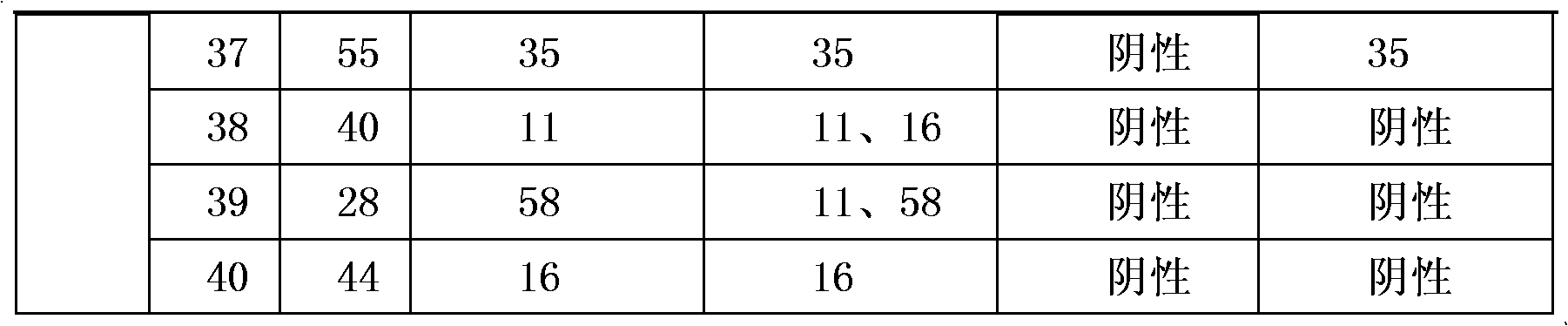
Medicament for treating HPV infection and application of medicament in preparation of medicament for treating HPV infection
A drug and pharmacy technology, applied in the field of biomedicine, can solve the problems that no human rabies vaccine treatment reports have been retrieved, and achieve the effect of not being easy to relapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: use rabies virus PV-2061 strain to produce human rabies vaccine
[0021] Rabies virus PV-2061 strain, inoculated in eukaryotic cells (including Vero cells, hamster kidney cells, human diploid cells, chicken embryo cells, preferably Vero cells), harvested virus liquid, inactivated, purified, added The pharmaceutically acceptable carrier is used to prepare rabies vaccine preparations for adults, and the dose is 0.5-10.5 IU / dose.
Embodiment 2
[0022] Embodiment 2: Production of human rabies vaccine with rabies virus PM strain
[0023] The PM strain of rabies virus is inoculated in eukaryotic cells (including Vero cells, hamster kidney cells, human diploid cells, chicken embryo cells, preferably human diploid cells), and the virus liquid is harvested, inactivated and purified. A pharmaceutically acceptable carrier is added to prepare a rabies vaccine preparation for adults, 0.5-10.5 IU / dose.
Embodiment 3
[0024] Embodiment 3: use rabies virus Vnukovo-32 strain to produce human rabies vaccine
[0025] Rabies virus Vnukovo-32 strain, inoculated in eukaryotic cells (including Vero cells, hamster kidney cells, human diploid cells, chicken embryo cells, preferably hamster kidney cells), harvested virus fluid, inactivated, and purified , adding a pharmaceutically acceptable carrier to prepare a rabies vaccine preparation for adults, 0.5-10.5 IU / dose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com