Synthetic method of lambdacyhalothrin hapten
A technology of kungfu pyrethroid and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., to achieve the effect of safe and feasible synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
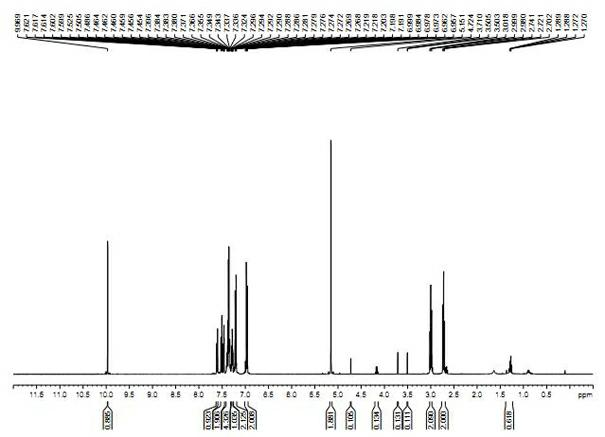
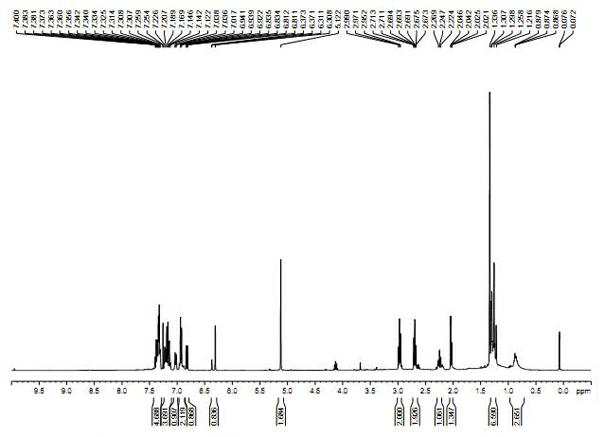
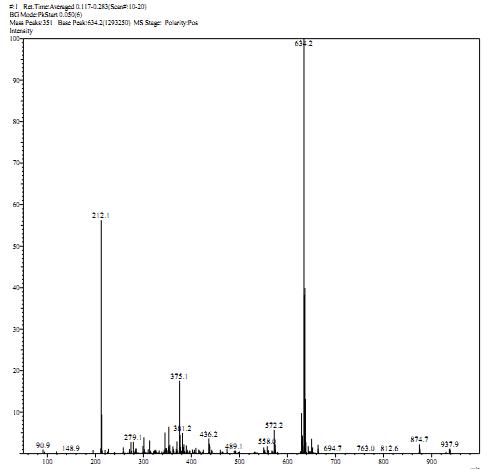
Image
Examples
Embodiment 1
[0032] (1) Synthesis of benzyl 4-hydroxyphenylpropionate (compound 1)
[0033] In a 100 mL round bottom flask, add 5.0 g (30.1 mmol) p-hydroxyphenylpropionic acid, 3.4 g (34.0 mmol) potassium bicarbonate (KHCO 3 ), 15mL N,N'-dimethylformamide (DMF), and then add 8.0g (46.8mmol) benzyl bromide (BnBr), and stir at room temperature overnight. After the reaction, the reaction solution was diluted with ethyl acetate, and then with saturated NaHCO 3 Wash 3 times, wash 2 times with distilled water, and finally wash 2 times with saturated saline. The organic phase was dried, concentrated, and purified by column chromatography to obtain 6.9 g of benzyl 4-hydroxyphenylpropionate, a colorless viscous liquid, with a yield of about 89.4%.
[0034] (2) Synthesis of 3-(dimethoxymethyl)bromobenzene (compound 2)
[0035] Add 7.4g (40.0mmol) m-bromobenzaldehyde, 17.0g (160.0mmol) trimethyl orthoformate, 74mg (0.4mmol) p-toluenesulfonic acid into a 100mL round bottom flask, then heat and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com