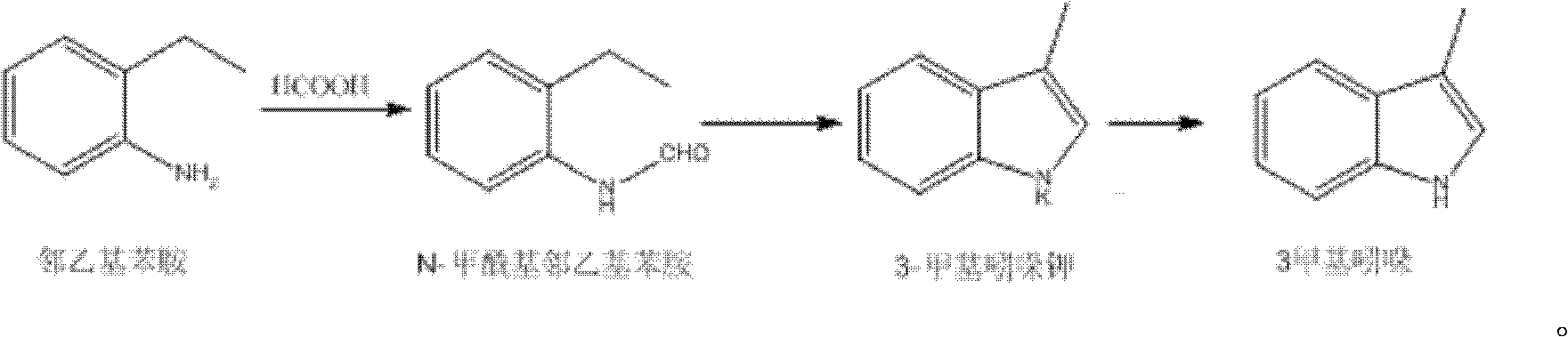
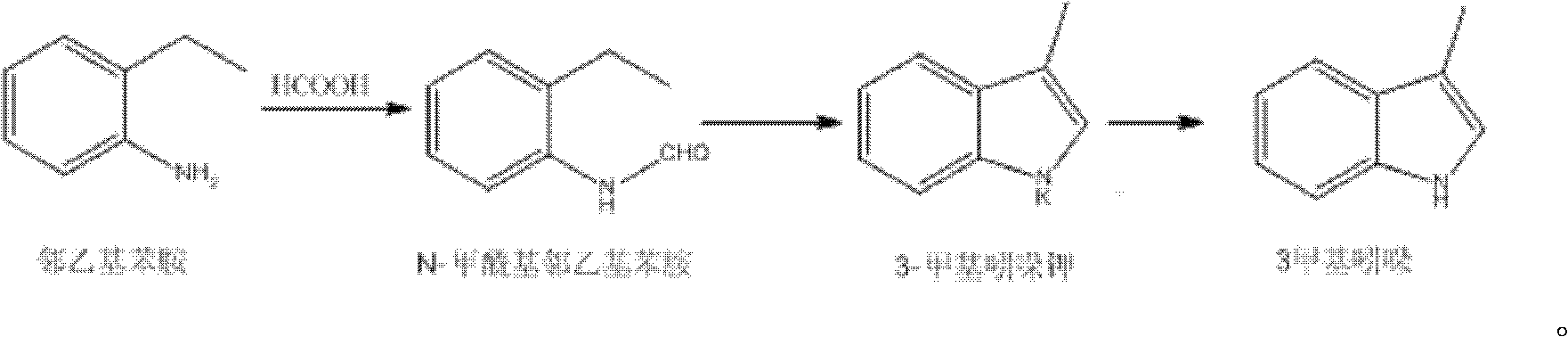Synthetic method of 3-methyl indole
A technology of methyl indole and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of complex reaction process, large toxic reagent, high price, etc., and achieve the effect of simple process, safe reaction reagent, and industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 96.8g of o-ethylaniline and 40.3g of formic acid aqueous solution with a volume fraction of 90% in a 250mL three-necked flask, heat to reflux for 3h, then place the reaction system overnight, evaporate the solvent under reduced pressure, then add 35mL of toluene and heat to dissolve. Cool to 10°C for crystallization for 6 hours, and filter with suction to obtain 105 g of N-formyl o-ethylaniline, m.p: 59-61°C, yield 89%.
[0015] Add 36g KOH and 100mL toluene into a 500mL three-necked flask and heat to reflux, slowly add a solution consisting of 72g N-formyl o-ethylaniline and 50mL toluene, dropwise for 3h, react for 2h after the dropwise addition, and then heat up and steam out Solvent until the temperature in the bottle reaches 320°C, then cool down naturally, add 50mL*3 water, stir and wash, dry to obtain 50.5g of 3-methylindole, m.p: 95-97°C, yield 80%.
Embodiment 2
[0017] Add 64.5g of o-ethylaniline and 32.3g of formic acid aqueous solution with a volume fraction of 90% in a 250mL three-necked flask, heat to reflux for 2h, then place the reaction system overnight, evaporate the solvent under reduced pressure, then add 25mL of toluene and heat to dissolve. Cool to 10°C and crystallize for 7 hours, filter with suction to obtain 70 g of N-formyl o-ethylaniline, m.p: 59-61°C, yield volume fraction 90%.
[0018] Add 24g KOH and 80mL toluene into a 500mL three-necked flask and heat it to reflux, slowly add a solution consisting of 48g N-formyl o-ethylaniline and 30mL toluene, dropwise for 2h, react for 2h after the dropwise addition, and then heat up and steam out Solvent until the temperature in the bottle reaches 320°C, then cool down naturally, add 50mL*3 water, stir and wash, dry to obtain 35 grams of 3-methylindole, m.p: 95-97°C, yield 81%.
Embodiment 3
[0020] Add 174g of o-ethylaniline and 58.1g of formic acid aqueous solution with a volume fraction of 90% in a 500mL three-necked flask, heat to reflux for 4 hours, then place the reaction system overnight, evaporate the solvent under reduced pressure, then add 50mL of toluene to dissolve, cool Crystallize at 10°C for 8 hours, and filter with suction to obtain 180 g of N-formyl o-ethylaniline, m.p: 59-61°C, yield 89%.
[0021] Add 43g KOH and 150mL toluene into a 500mL three-necked flask and heat to reflux, then slowly add a solution consisting of 129g N-formyl o-ethylaniline and 90mL toluene, the time for the dropwise addition is 4h, after the dropwise addition, react for 3h, then heat up and steam out Solvent until the temperature in the bottle reaches 320°C, then cool down naturally, add 100mL*3 water, stir and wash, dry to obtain 110g of 3-methylindole, m.p: 95-97°C, yield 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com