Intermediate for preparing eribulin, and preparation method thereof
A chiral center and configuration technology, applied in the field of intermediates for the preparation of eribulin, can solve the problems of difficult purification process, hazardous reagents, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
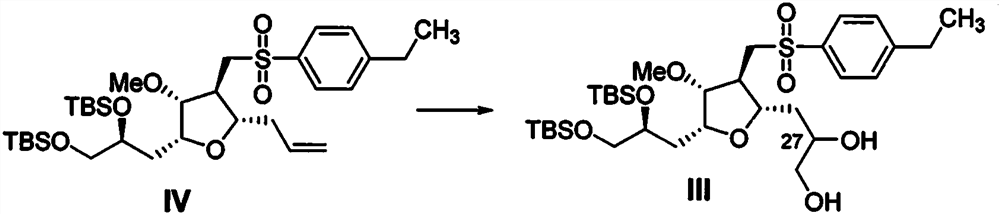
[0048] Embodiment 1: preparation compound III
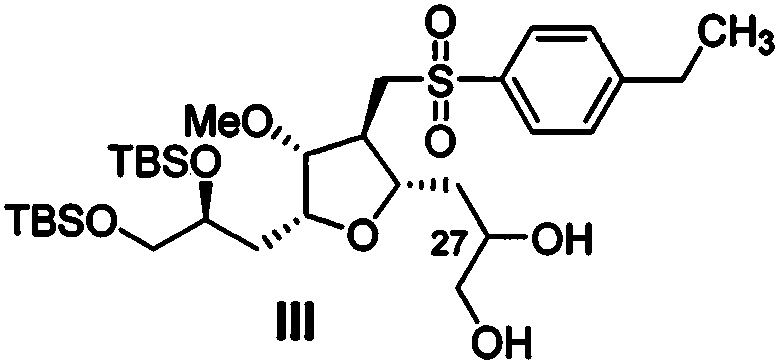
[0049] At 0°C, compound IV (20g, 31.90mmol, 1eq.) was dissolved in a mixed solvent of acetone (200mL) and water (50mL), and potassium osmate (117mg) and NMO (4.93g) were added successively, and the reaction was carried out at 20 Stirring at ℃ for 6 h, TLC showed that the conversion of the raw material was complete, and the reaction was quenched by adding an aqueous solution of sodium thiosulfate. After extraction with ethyl acetate, the organic phase was concentrated and purified by column chromatography to obtain 19.95 g of compound III.
[0050] MS (ESI) m / z: 661 (M+H + ).
[0051] 1H NMR (400MHz, Chloroform-d) δ7.74(d, J=7.9Hz, 2H), 7.34(d, J=7.9Hz, 2H), 3.92-3.76(m, 2H), 3.76-3.60(m, 5H), 3.61-3.45(m, 2H), 3.44-3.35(m, 2H), 3.31(d, J=6.5Hz, 3H), 3.00(dd, J=7.5, 3.9Hz, 2H), 2.68(q , J=7.6Hz, 2H), 2.50(q, J=6.1, 4.6Hz, 1H), 2.02-1.84(m, 2H), 1.87-1.51(m, 7H), 1.20(t, J=7.6Hz, 3H), 0.80(dd, J=3.8, 1.0Hz, 18H), 0.00(s, 6H),...
Embodiment 2
[0052] Embodiment 2: preparation compound III
[0053] At 20°C, compound IV (20g, 31.90mmol, 1eq.) was dissolved in a mixed solvent of tert-butanol (200mL) and water (50mL), and osmium tetroxide (95mg) and K 3 Fe(CN) 6 (15.75 g), the reaction was stirred at 40° C. for 2 h, TLC showed that the conversion of the starting material was complete, and an aqueous solution of sodium thiosulfate was added to quench the reaction. After extraction with ethyl acetate, the organic phase was concentrated and purified by column chromatography to obtain 19.02 g of compound III.
[0054] MS (ESI) m / z: 661 (M+H + ).
[0055] 1 H NMR (400MHz, Chloroform-d) δ7.74(d, J=7.9Hz, 2H), 7.34(d, J=7.9Hz, 2H), 3.92-3.76(m, 2H), 3.76-3.60(m, 5H), 3.61-3.45(m, 2H), 3.44-3.35(m, 2H), 3.31(d, J=6.5Hz, 3H), 3.00(dd, J=7.5, 3.9Hz, 2H), 2.68(q , J=7.6Hz, 2H), 2.50(q, J=6.1, 4.6Hz, 1H), 2.02-1.84(m, 2H), 1.87-1.51(m, 7H), 1.20(t, J=7.6Hz, 3H), 0.80(dd, J=3.8, 1.0Hz, 18H), 0.00(s, 6H), -0.04(s, 6H).
Embodiment 3
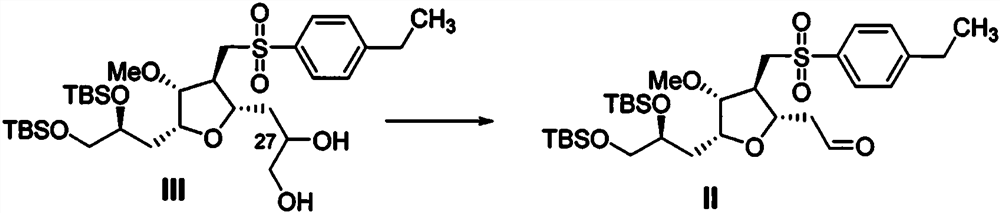
[0056] Embodiment 3: preparation compound II
[0057] At 0°C, compound III (18g, 27.23mmol, 1eq.) was dissolved in a mixed solvent of tetrahydrofuran (200mL) and water (200mL), sodium periodate (6.99g) was added, and the reaction was stirred at 25°C for 2h, TLC showed that the conversion of the raw material was complete, the reaction solution was filtered through celite, and the filter cake was washed with ethyl acetate. The filtrate was concentrated and purified by column chromatography to obtain 16.4 g of compound II. HPLC purity 99.3%.
[0058] MS (ESI) m / z: 629 (M+H + ).
[0059] 1 H NMR (400MHz, Chloroform-d) δ9.63 (t, J = 1.4Hz, 1H), 7.83-7.60 (m, 2H), 7.39-7.25 (m, 2H), 3.94-3.81 (m, 2H), 3.78-3.63(m, 2H), 3.49(dd, J=10.2, 5.5Hz, 1H), 3.38(dd, J=10.2, 5.5Hz, 1H), 3.29(s, 3H), 3.22(dd, J= 14.1, 5.1Hz, 1H), 3.00 (dd, J = 14.1, 8.9Hz, 1H), 2.81 (ddd, J = 17.5, 6.5, 1.8Hz, 1H), 2.78 (q, J = 7.6Hz, 2H), 2.70(ddd, J=17.5, 5.9, 1.2Hz, 1H), 2.42(ddd, J=8.9, 5.0, 1.2Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com