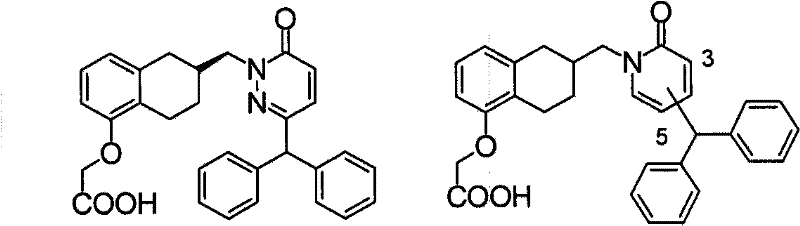
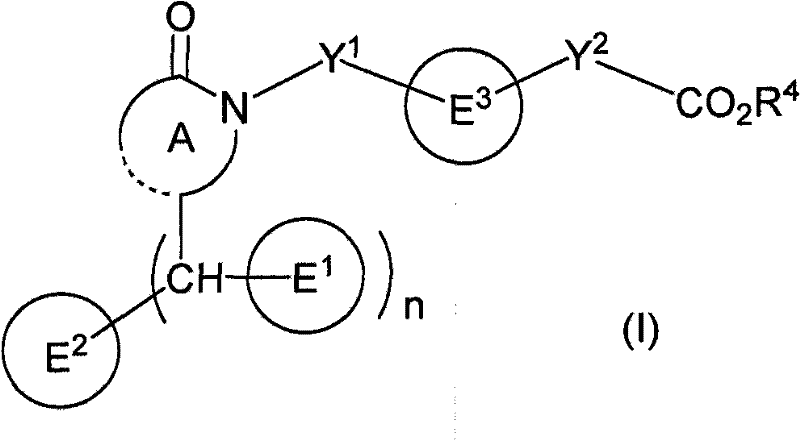
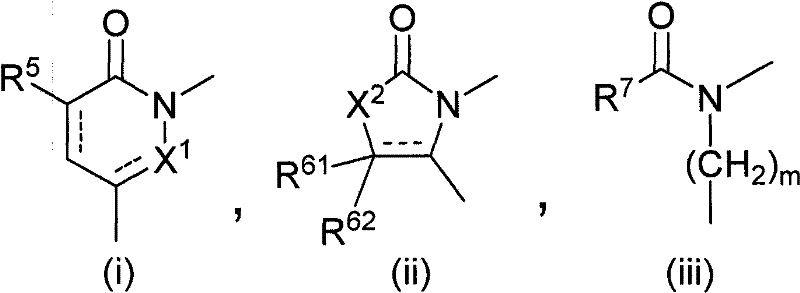
Polycyclic acid compounds useful as crth2 antagonists and antiallergic agents
A compound, C1-C6 technology, applied in the direction of the active ingredients of heterocyclic compounds, the preparation of organic compounds, allergic diseases, etc., can solve the problems of not mentioned etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0266] Hereinafter, the present invention is further elucidated and described with reference to examples, but the present invention is not limited to these examples. In this regard, new materials included in the starting materials were used in the examples, and the preparation methods for obtaining starting materials from known materials are described in "Preparations".
[0267] The reactions used to prepare the compound (I) of the present invention in each of "Preparations" and "Examples" are described in more detail below. The present invention should not be limited by the following "Preparations" and "Examples" in any way.
preparation example 1
[0269] To a solution of 5-(diphenylmethyl)-1-ethoxycarbonylmethyl-2(1H)-pyridone (565 mg) in EtOH was added dropwise 1M aqueous NaOH (4.9 mL) at room temperature, and the mixture was stirred at room temperature. The mixture was left for 1 hour. The resulting mixture was diluted with water (25 mL) and acidified with 1M aqueous HCl (10 mL). The precipitate was collected by filtration and washed with water (10 mL) to obtain 5-(diphenylmethyl)-1-carboxymethyl-2(1H)-pyridone (472 mg) as colorless crystals.
[0270] MS (ESI, m / z): 320 (M+H) + .
preparation example 2
[0272] To a solution of (2E,2′E)-3,3′-(1,3-phenylene)diethylacrylate (27.9 g) in a mixture of EtOH (300 mL) and THF (200 mL) at 0° C. 1M NaOH aqueous solution (92 mL) was added. The reaction mixture was stirred at the same temperature for 5 hours. The organic solvent of the reaction mixture was evaporated in vacuo and the obtained liquid was washed with EtOAc. The aqueous layer was neutralized with 1M aqueous HCl (92 mL), and the precipitate was collected by filtration. The precipitate was recrystallized twice from EtOH-water to obtain (2E)-3-{3-[(1E)-3-ethoxy-3-oxopropyl-1-en-1-yl]phenyl} Acrylic acid (10.5 g) as colorless crystals.
[0273] MS (ESI, m / z): 245 (M-H) - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com