Diffractive ring mangiferin in mango leaf and new application of mango leaf extract containing diffractive ring mangiferin
A technology of mango leaf and mangiferin, applied in the field of mango leaf extract, can solve the problems such as inability to be widely used, induce cancer, etc., and achieve the effects of preventing wrinkles, strong antioxidant activity, and inhibiting the chain reaction of oxygen free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of Active Ingredient Monomer
[0032] 5 kg of mango leaves were reflux extracted twice with 9 times the amount of ethanol, each time for 3 hours, the filtrates were combined, and the solvent was recovered; dried in a vacuum oven at 50°C to obtain 1.163 kg of extract.
[0033] Take 600g of the above-mentioned extract, add 5L of distilled water to dissolve, extract three times with 5L of ethyl acetate to obtain ethyl acetate layer and water layer respectively; the water layer part contains a large amount of precipitation, take 4 / 5 of the volume of the suspension in the water layer, and concentrate under reduced pressure to 2L, centrifuged, filtered, and the supernatant was treated with a macroporous adsorption resin (H2O→95%EtOH) to obtain 84g of 95% ethanol eluate.
[0034] Take 72 g of the above-mentioned 95% ethanol eluate and perform silica gel column chromatography [chloroform-methanol (10:1 → 5:1) → chloroform-methanol-water (7:3:1 → 6:4:1, lower layer) ...
Embodiment 2
[0036] Confirmation of the structure of the active ingredient
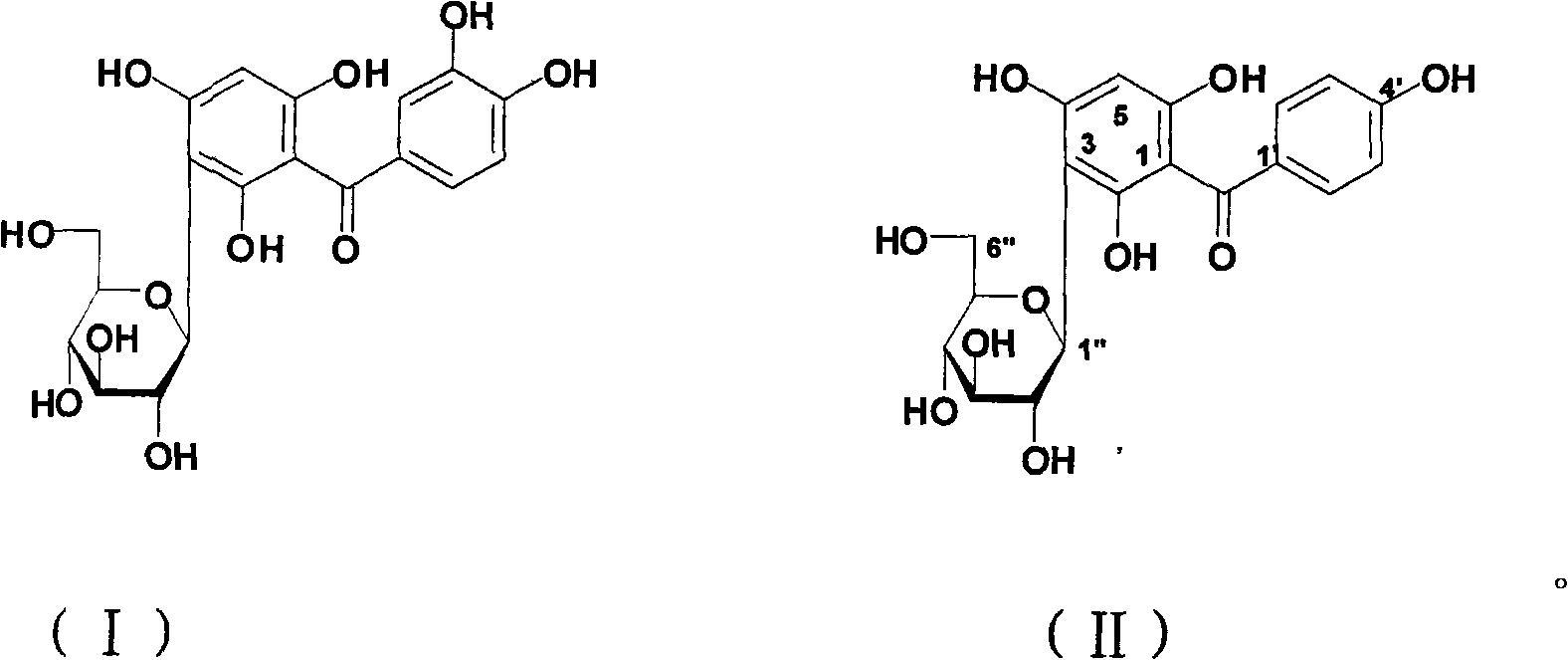
[0037] The structure of the two compounds obtained in Example 1 (i.e., split-mangiferin A and split-ring mangiferin B) was confirmed (entrusted to Tianjin WuXi Kangde New Drug Development Co., Ltd. Analysis and Testing Center, 400MHz NMR), and the analysis results showed The structures of these compounds are consistent with those reported in the literature, as follows:
[0038] Split ring mangiferin A, colorless needle crystal, NMR data are as follows:.
[0039] 1H-NMR (DMSO-d6, 400MHz): 7.16 (1H, d, J = 2.0Hz, 2'-H), 7.06 (1H, dd, J = 2.0, 8.0Hz, 5'-H), 6.74 (1H , d, J=8.0Hz, 6′-H), 5.95 (1H, s, 5-H), 4.61 (1H, d, J=9.6Hz, 1″-H), 3.63 (1H, br.d, ca.12Hz, 6″-H), 3.55(1H, dd, J=9.6, 9.6Hz, 2″-H), 3.51(1H, dd, J=3.2, 11.6Hz, 6″-H), 3.22( 1H, m, overlapped, 3″-H), 3.22 (1H, m, overlapped, 4″-H), 3.22 (1H, m, overlapped, 6″-H, 5″-H).
[0040] 13C-NMR (DMSO-d6, 100MHz): 60.3 (6″-C), 69.4 (4″-C), 71.9 (2″-C), 74.6 (1″...
Embodiment 3
[0048] Antioxidant Experimental Research on Compounds of the Invention
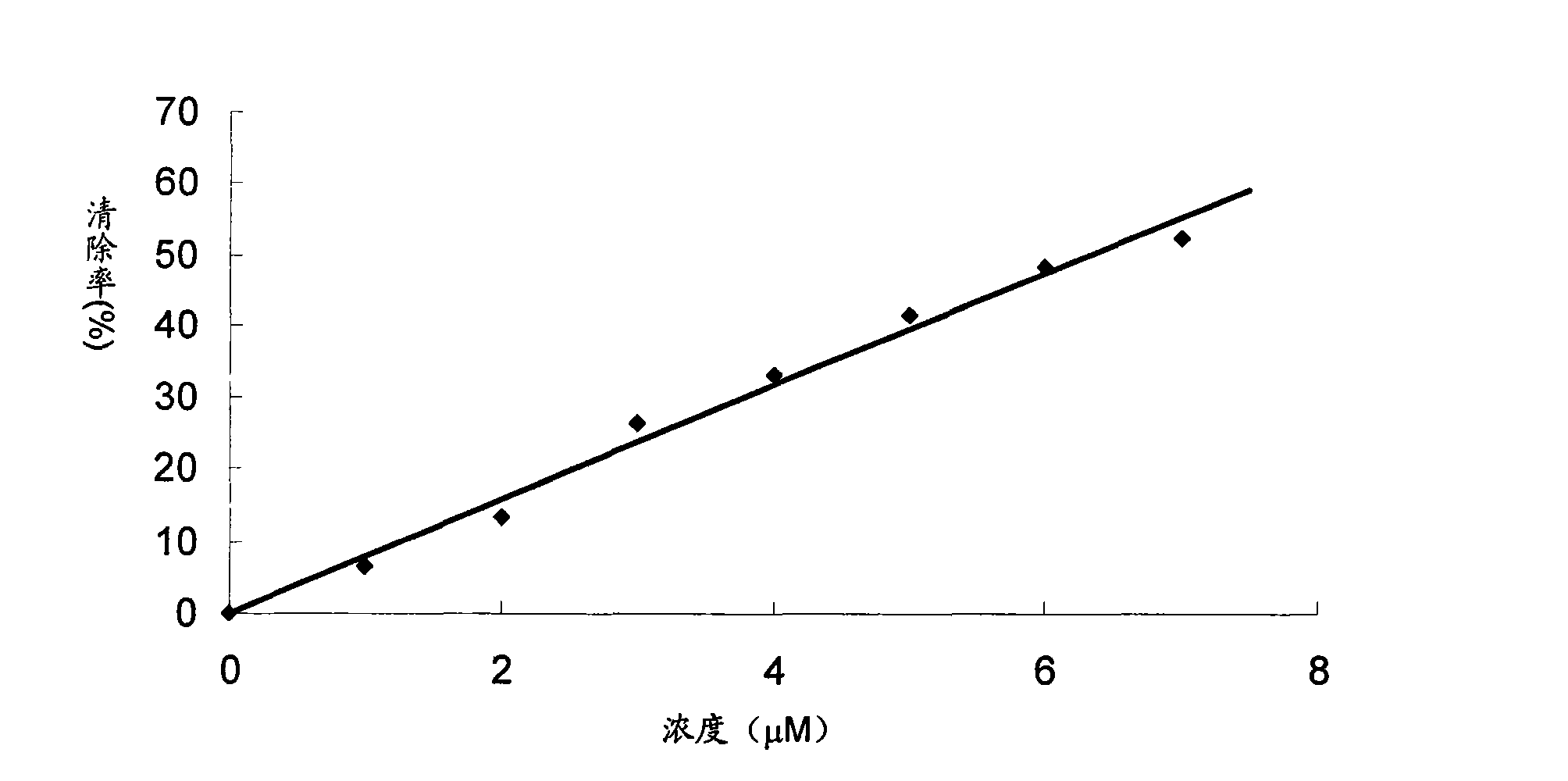
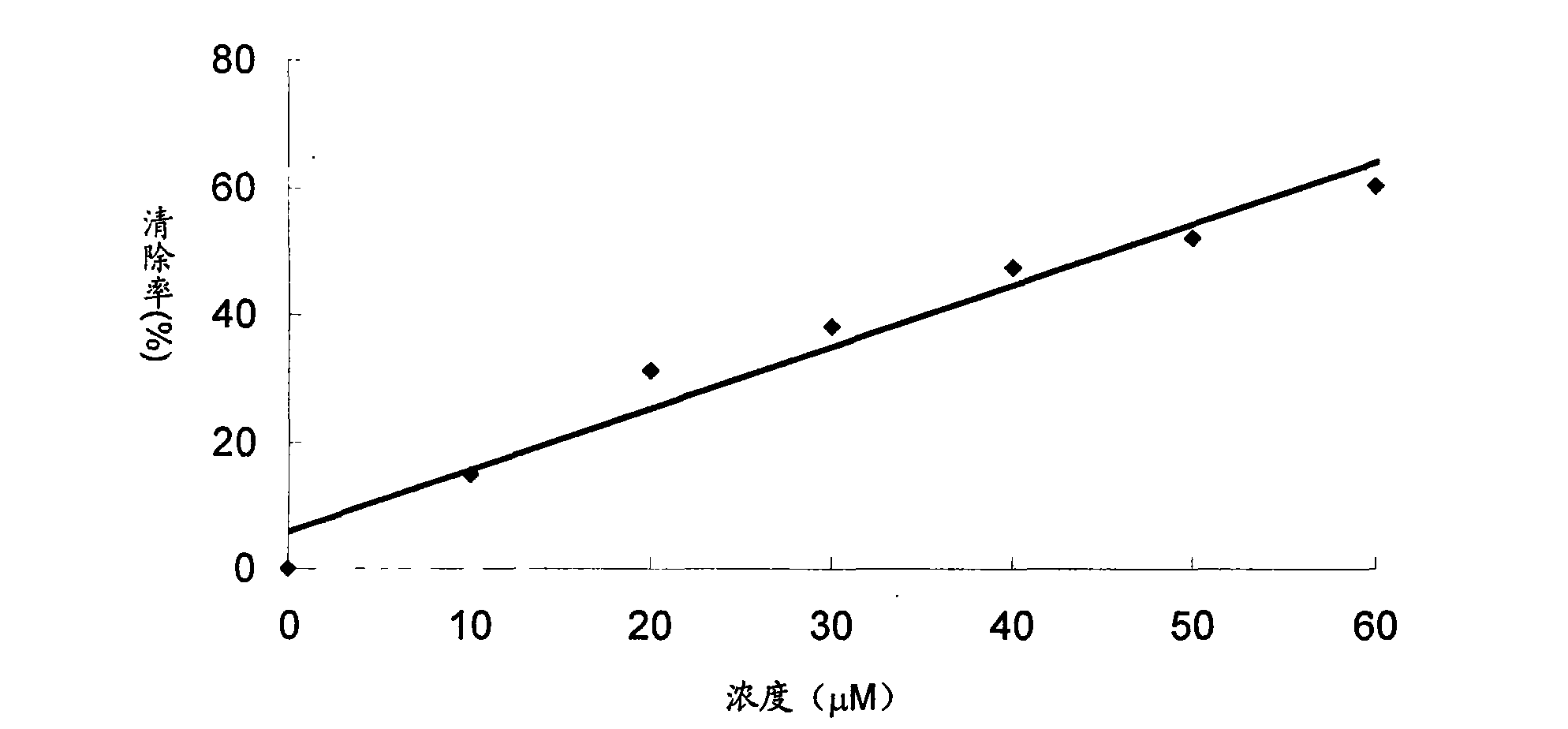
[0049] Superoxide dismutase-like free radical elimination assay
[0050] Sample group: dissolve the sample in 2.5% dimethyl sulfoxide aqueous solution, add the reaction solution, the reaction solution is 40mM carbonic acid buffer solution (pH 10.2), which contains 100uM xanthine, 100uM ethylenediaminetetraacetic acid dipotassium salt, 25uM NBT (nitroblue tetrazolium), 0.005% fetal bovine serum, about 1.8mU / mL xanthine oxidase, adjust the total volume to 3ml, react in a 37 degree water bath for 20 minutes, add 0.1mL 6mM copper chloride solution to make the reaction Stop, measure the ultraviolet absorption of each sample at a wavelength of 560nm, and calculate the ability to eliminate free radicals according to the following method.
[0051] Wherein, the samples are sample 1-ethanol crude extract; sample 2-component 7-2; sample 3-semangiferin A; sample 4-mangiferin B; sample 5-tetramethylcyclohexyl olefin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com