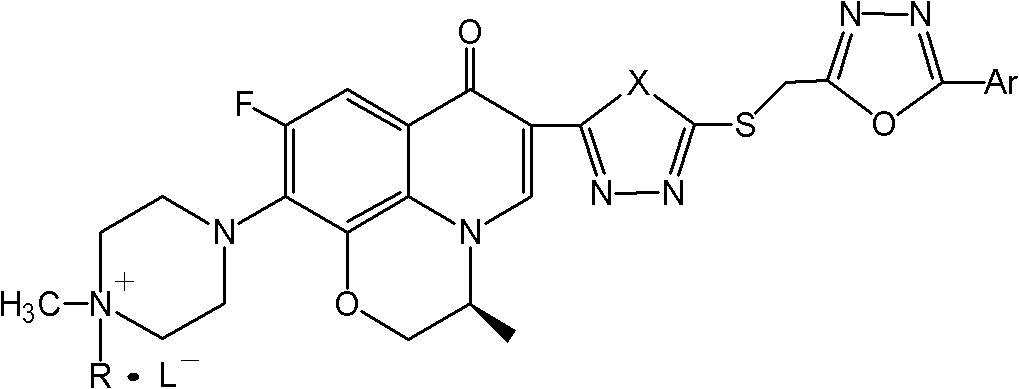
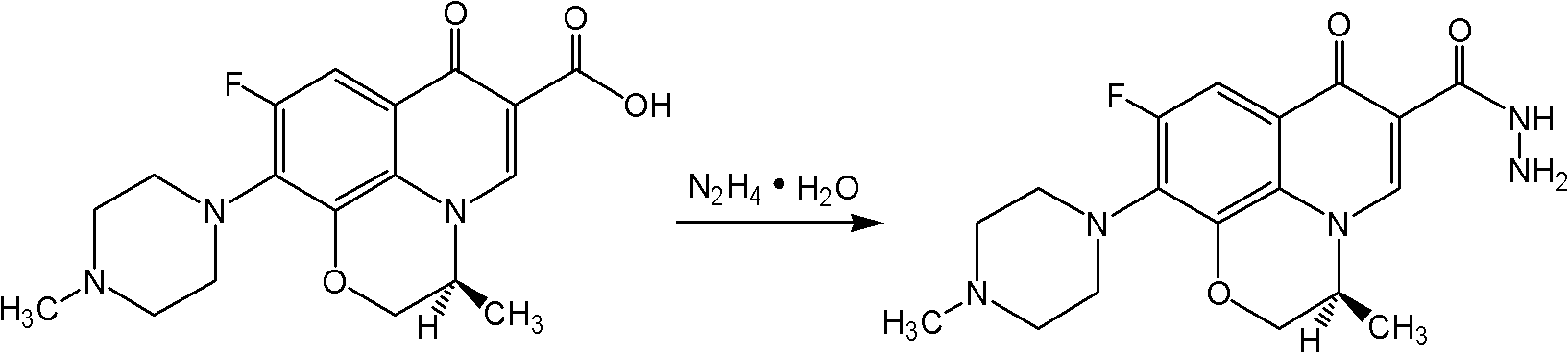
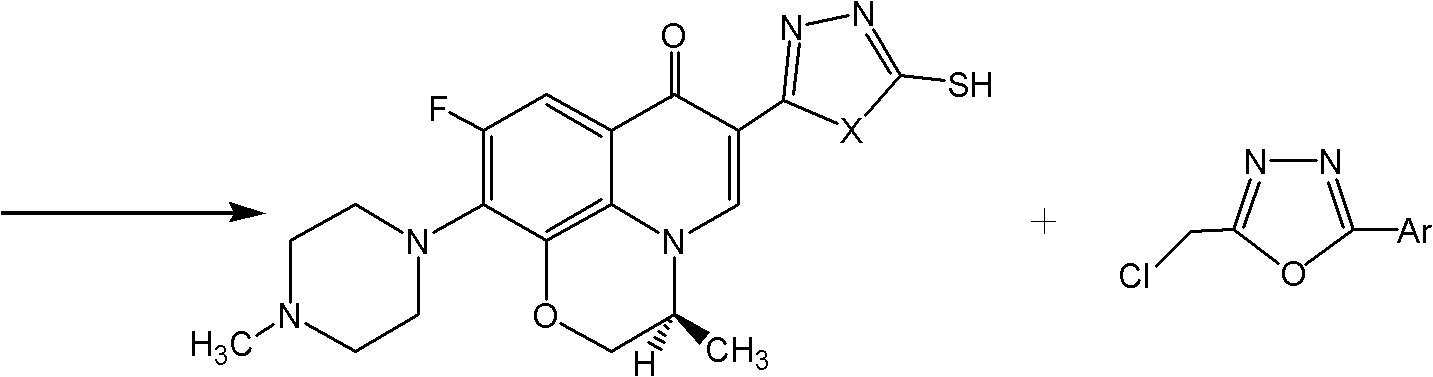
Levorotatory fluoroquinolone C3 diazole methyl sulfide quaternary ammonium salt, preparation method and application thereof
A technology of L-fluoroquinolone and fluoroquinolone, which is applied in the field of medicine, can solve the problem that the research on isosteres has not yet been reported, and achieves the effects of strong in vitro cytotoxicity and strong antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (S)-8,1-[1,2-(oxypropyl)]-6-fluoro-7-(4,4-dimethylpiperazin-1-yl)-3-[5-(5- Phenyl-1,3,4-oxadiazol-2-methylthio)-1,3,4-oxadiazol-2-yl]-4(1H)-quinolinone iodide, the structural formula is:
[0058]
Embodiment 2
[0060] (S)-8,1-[1,2-(oxypropyl)]-6-fluoro-7-(4,4-dimethylpiperazin-1-yl)-3-[5-(5- p-methoxyphenyl-1,3,4-oxadiazol-2-methylthio)-1,3,4-oxadiazol-2-yl]-4(1H)-quinolinone iodide, structural formula for:
[0061]
Embodiment 3
[0063] (S)-8,1-[1,2-(oxypropyl)]-6-fluoro-7-(4,4-dimethylpiperazin-1-yl)-3-[5-(5- p-Tolyl-1,3,4-oxadiazol-2-methylthio)-1,3,4-oxadiazol-2-yl]-4(1H)-quinolinone iodide, the structural formula is:
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com