Combination therapy using an anti-egfr agent(s) and igf-1r specific inhibitors
A technology for IGF-1R, an inhibitor, applied in combination with erlotinib, a receptor tyrosine kinase inhibitor, for the treatment of non-small cell lung cancer and other cancers (e.g. field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0217] Example 1 - Correlation between activation of EGFR and IGF1R and efficacy of MK-0646 / erlotinib combination:
[0218] SUMMARY: Activation of multiple receptor tyrosine kinases in cells contributes to resistance to erlotinib. Indeed, activation of EGFR and cMET has been observed in clinical samples from erlotinib-resistant patients.
[0219] Methods: To identify tumors that responded to the MK-0646 / erlotinib combination, the phosphorylation status of different RTKs in a panel of lung cancer cell lines was evaluated. As shown in Figure 1, activated EGFR and IGFlR levels were quantified in a panel of 10 lung cancer cell lines. A few cell lines represented by NCI-H2122 and NCI-H322M exhibited high levels of P-IGF1R and P-EGFR, while the EGFR mutant cell line HCC827 exhibited high levels of P-EGFR with little or no IGF1R activation.
[0220] Briefly, all NSCLC cell lines were obtained from ATCC and maintained in DMEM or RPMI with 10% FBS as directed by ATCC. Approximately...
Embodiment 2
[0221] Example 2 - Inhibition of PI3K and RAS-MAPK Signaling by the MK-0646 / Erlotinib Combination
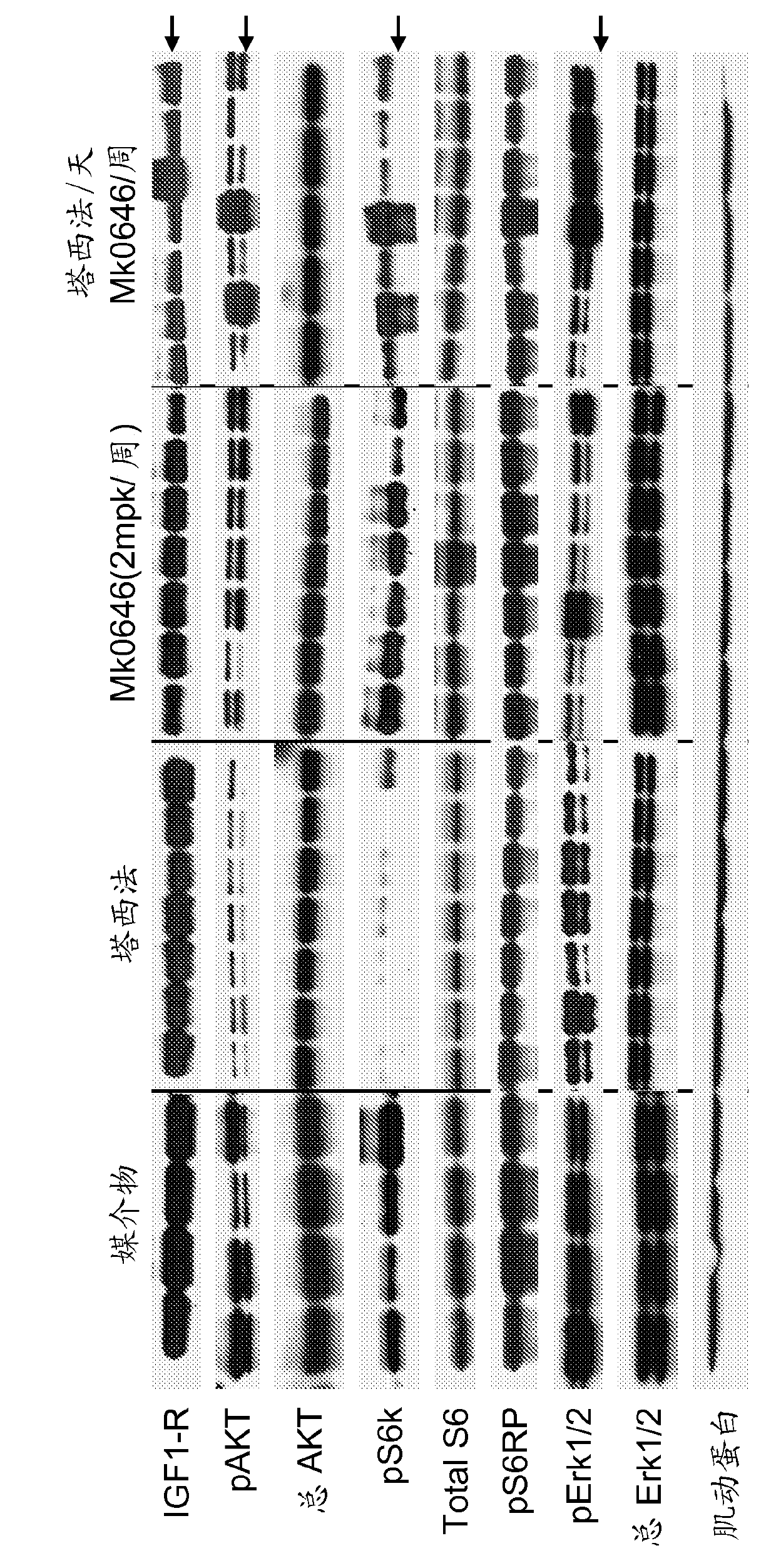
[0222] SUMMARY: To test the inhibitory effect of these RTKs on PI3K and RAS-MAPK pathway activity, the phosphorylation status of key nodes in the pathway was measured. Such as figure 2 As shown, combined inhibition of EGFR and IGF1R can more effectively block the PI3K pathway, as measured by a substantial reduction in P-S6RP and P-S6K in the NCI-H2122 and NCI-H322M cell lines, which express High levels of both receptors. In cell lines with low levels of P-EGFR and P-IGF1R, this synergistic inhibition of PI3K signaling was not observed (A427 is shown as an example). In other cell lines, similar results were obtained (data not shown).
[0223] Methods: For Western blot analysis, total protein lysates from cells (~300,000) cultured in 6-well plates and stained with Deforolimus (10 nM) or MK- 0646 (10ng / ml) or a combination of the two were treated for 4 hours. Samples were sub...
Embodiment 3
[0224] Example 3 - Functional Effects of Inhibiting EGFR and IGF-1R Signaling
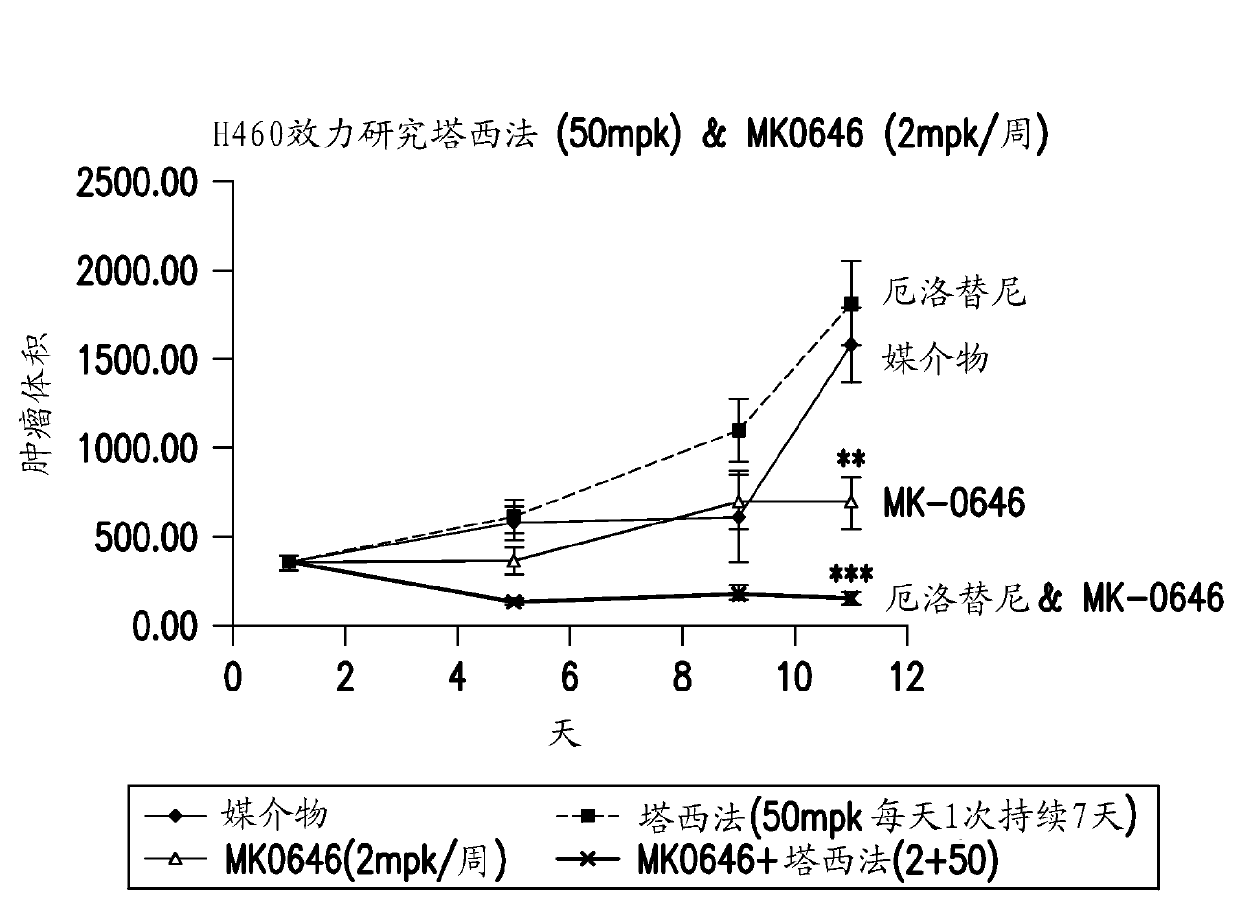
[0225] Summary: To test the functional effect of inhibiting EGFR and IGF1R signaling, growth inhibition under adherent (2D) and non-adherent (3D) conditions was evaluated. Under adherent growth conditions, no significant growth inhibition was observed in MK-0646-treated cell lines. This is consistent with previous experiments (data not shown). To test the effect of this combination under 3D non-adhesive conditions, the inventors developed an ultra-low-adhesion plate-based proliferation assay. Only 7 / 10 cell lines grew measurably when cultured under non-adherent conditions and were used for sensitivity assessment. Under non-adherent conditions, NCI-H2122 cells exhibited a substantial increase in sensitivity to the erlotinib / MK-0646 combination. On the other hand, A427 cells with low levels of P-IGF1R and EGFR showed no significant growth inhibition under 2D or 3D growth conditions.
[0226] Meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com