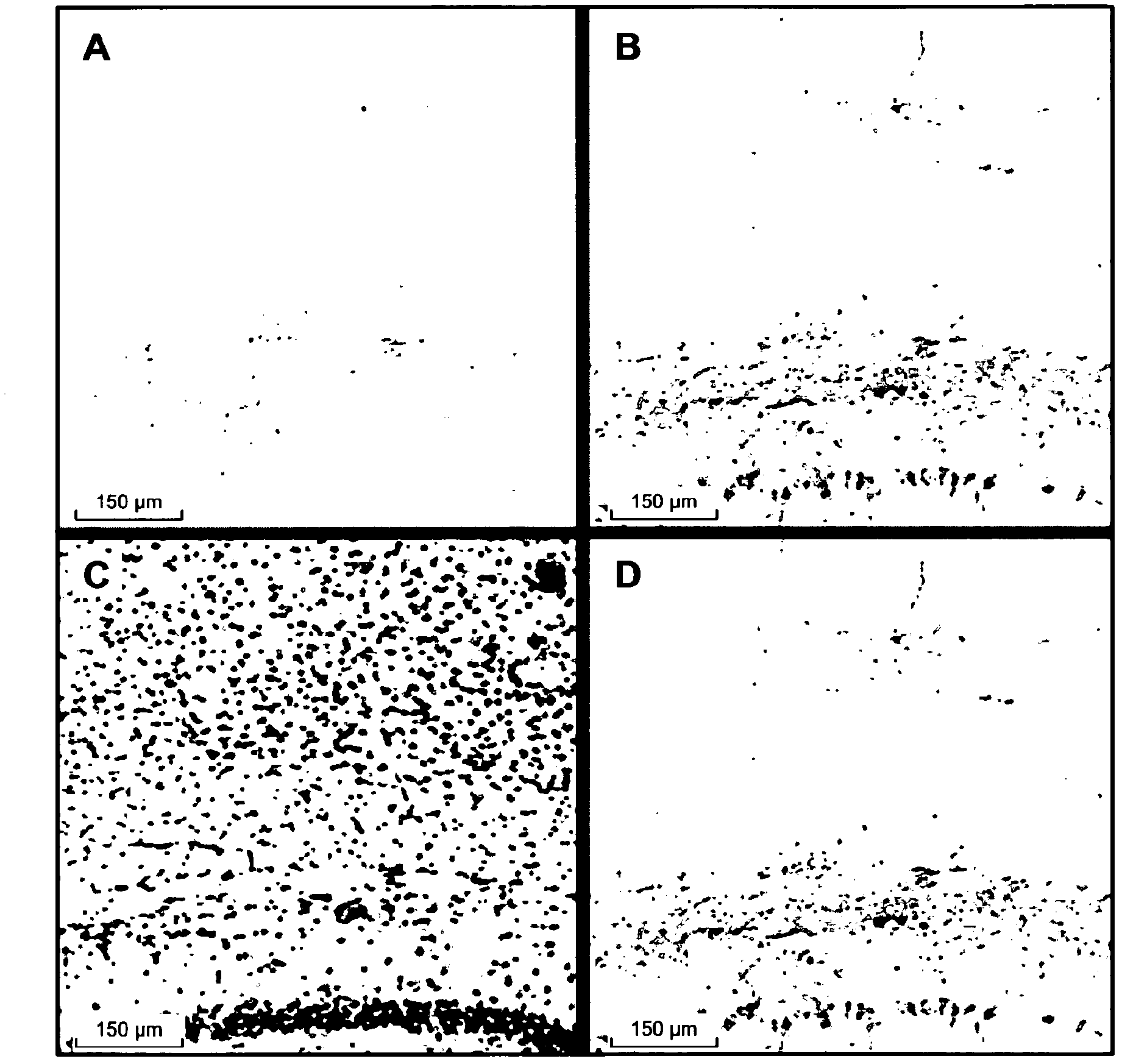
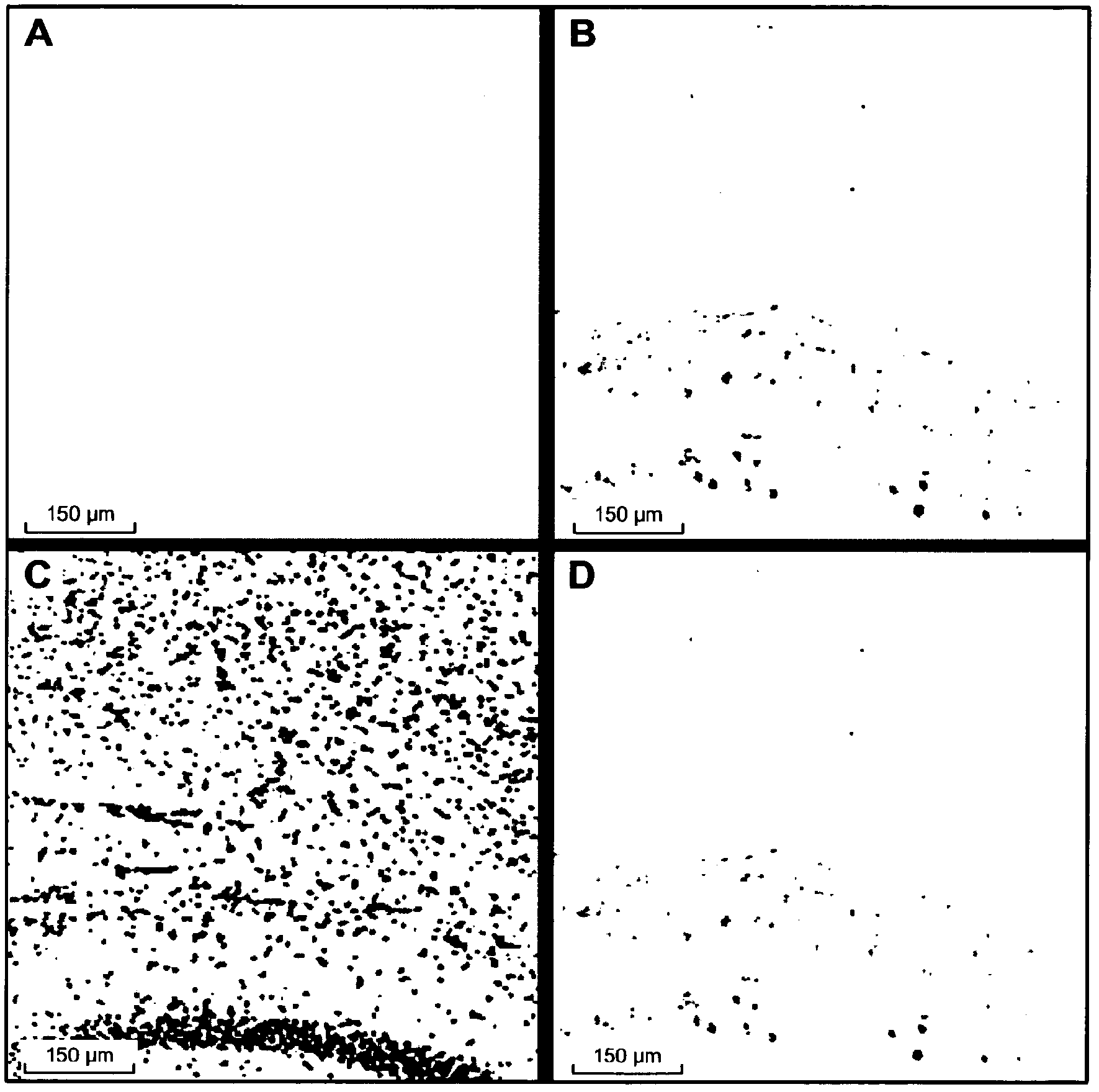
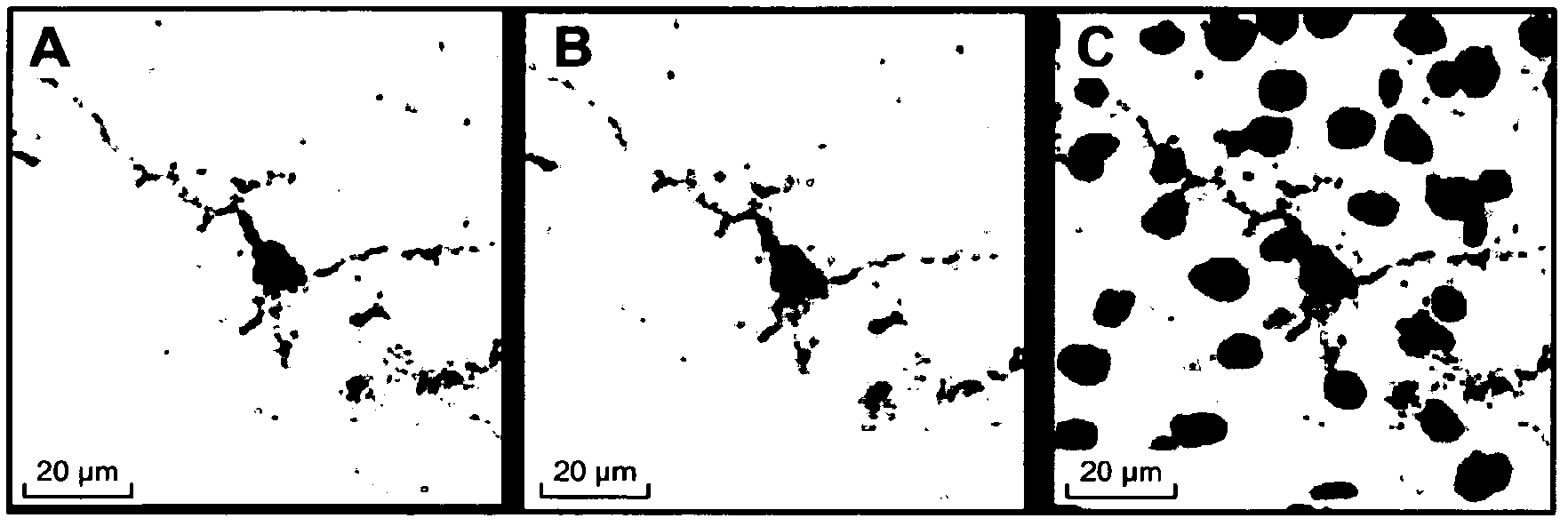
Use of an anti-tau ps422 antibody for the treatment of brain diseases
An antibody and phosphorylation technology, applied in the direction of antibodies, tumor rejection antigen precursors, antibody mimics/scaffolds, etc., can solve unclear problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0233] Preparation and purification of antibodies
[0234] a) Antibody production
[0235] Mice use SEQ ID NO: 9 (Ser-Ile-Asp-Met-Val-Asp-Ser (PO 3 h 2 )-Pro-Gln-Leu-Ala-Thr-Leu-Ala-Asp), which corresponds to amino acids 416-430 of the longest human isoform of Tau. To allow direct coupling to KLH via thiols, a cysteine was added to the Tau fragment at the N-terminus. Subsequent immunization schemes, fusion and cloning and screening for anti-TaupS422 specific antibodies are described in EP 1 876 185 .
[0236] b) Purified clones 2.10.3, 2.20.4 and 5.6.11
[0237] Cell-free hybridoma culture supernatant (250-300 ml) was loaded onto a 25 ml MEP Hypercell column (pall Biosciences) equilibrated with 50 mM TrisCl pH 8.0. After washing with equilibration buffer, the antibody was eluted with 30 mM sodium citrate, 100 mM NaCl pH 4.1. Antibody-containing fractions were pooled and then dialyzed overnight at 4° C. in Spectra-Por 6-8000 dialysis tubing against 5 liters of 10 mM Tri...
Embodiment 2
[0240] Preparation of Tau, Tau pS422, Aggregated Tau and Aggregated Tau pS422
[0241] Contains N-terminal (His) 6 -SUMO fusion-tagged Tau was expressed in Escherichia coli (E.coli) and passed through ion-exchange chromatography on HiTrapQ (GE Healthcare, Switzerland), followed by affinity affinity on Ni-NTA Sepharose (Qiagen, Switzerland) Chromatographic purification. The fusion tag was then cleaved by digestion with SUMO protease (Invitrogen, The Netherlands), followed by a second Ni-NTASepharose chromatography step to remove the fusion tag.
[0242] Tau-pSer422 was prepared by incubating Tau with ERK2 protein kinase. The molar ratio of ERK2:Tau (ca:1:10) was selected by adding 1 mM MgCl at 37 °C 2 and 2 mM ATP in 10 mM TrisCl pH 8.0 overnight to produce maximal phosphorylation at S422. This was then assumed to represent stoichiometric phosphorylation at position 422. Phosphorylation was checked by western blotting using an in-house monoclonal antibody specific for pS42...
Embodiment 3
[0245] Anti-Tau pS422 mAb is highly selective for Tau phosphorylated at S422
[0246] a) Peptide synthesis
[0247] Peptide synthesis was performed on an automated peptide synthesizer using Fmoc chemistry. In repeated cycles, the peptide sequence is assembled by sequential coupling of the corresponding Fmoc-amino acids. In each coupling step, the N-terminal Fmoc-group was removed by treating the resin with 20% piperidine in N-methylpyrrolidone. Couplings were performed using Fmoc-protected amino acids (1 mmol) activated by HBTU / HOBt (1 mmol each) and DIPEA (2 mmol) in DMF. After each coupling step, unreacted amino groups were capped by treatment (10 min shaking) with a mixture of acetic acid (0.5M), DIPEA (0.125M) and HOBt (0.015M) in NMP. Between steps, the resin was washed with N-methylpyrrolidone and DMF. Incorporation of sterically hindered amino acids is accomplished by automatic double coupling. For this purpose, the resin was treated twice with 1 mmol of the activa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com