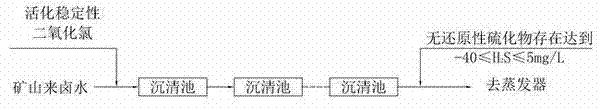
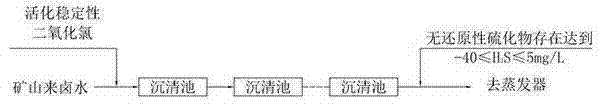
Process for removing sulfides from brine
A technology of sulfide and brine, applied in the direction of alkali metal chloride, etc., can solve the problem that sulfide cannot be removed fundamentally, achieve the effect of improving product quality, smooth process process, and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A process for removing sulfides in brine, comprising the following process steps:
[0021] A, stable chlorine dioxide and chlorine dioxide activator are added to the water of different containers respectively and dissolve, wherein the weight ratio of stable chlorine dioxide and water and the weight ratio of chlorine dioxide activator and water are 1: 3;
[0022] B, the stable chlorine dioxide solution in the step A and the chlorine dioxide activator solution are added in the activation tank, fully mixed, then placed for 10 minutes, fully reacted, and the stable chlorine dioxide solution was activated to obtain Activated stabilized chlorine dioxide liquid;
[0023] C. Mix the raw brine with the activated stable chlorine dioxide liquid, and then enter the sedimentation tank to remove gaseous H 2 S and combined H 2 Substance S, the amount of activated stable chlorine dioxide liquid is based on the gaseous H in the brine 2 S and combined H 2 The content of S substance ...
Embodiment 2
[0030] A process for removing sulfides in brine, comprising the following process steps:
[0031] A, stable chlorine dioxide and chlorine dioxide activator are added to the water of different containers respectively and dissolve, wherein the weight ratio of stable chlorine dioxide and water and the weight ratio of chlorine dioxide activator and water are 1: 7;
[0032] B, the stable chlorine dioxide solution in the step A and the chlorine dioxide activator solution are added in the activation tank, fully mixed, then placed for 25 minutes, fully reacted, and the stable chlorine dioxide solution was activated to obtain Activated stabilized chlorine dioxide liquid;
[0033] C. Mix the raw brine with the activated stable chlorine dioxide liquid, and then enter the sedimentation tank to remove gaseous H 2 S and combined H 2 Substance S, the amount of activated stable chlorine dioxide liquid is based on the gaseous H in the brine 2 S and combined H 2 The content of S substance ...
Embodiment 3
[0040] A process for removing sulfides in brine, comprising the following process steps:
[0041] A, stable chlorine dioxide and chlorine dioxide activator are added to the water of different containers respectively and dissolve, wherein the weight ratio of stable chlorine dioxide and water and the weight ratio of chlorine dioxide activator and water are 1: 5;
[0042] B, the stable chlorine dioxide solution in the step A and the chlorine dioxide activator solution are added in the activation tank, fully mixed, then placed for 17.5 minutes, fully reacted, and the stable chlorine dioxide solution was activated to obtain Activated stabilized chlorine dioxide liquid;
[0043] C. Mix the raw brine with the activated stable chlorine dioxide liquid, and then enter the sedimentation tank to remove gaseous H 2 S and combined H 2 Substance S, the amount of activated stable chlorine dioxide liquid is based on the gaseous H in the brine 2 S and combined H 2 The content of S substanc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com