Method for catalytic synthesis of tributyl citrate by utilizing immobilized p-toluenesulfonic acid
A technology of tributyl citrate and p-toluenesulfonic acid, which is applied in the chemical industry, can solve the problems of complex post-treatment process, many catalyst side reactions, complex preparation process, etc., achieve easy recovery and reuse, and improve the efficiency of esterification reaction , The effect of simplifying the post-processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Preparation of immobilized p-toluenesulfonic acid
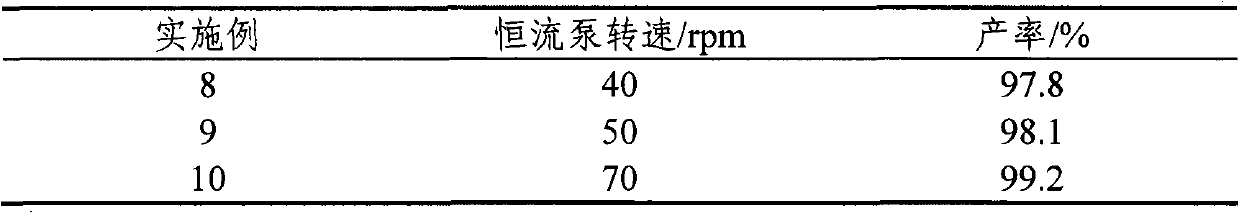
[0023] Add 2.5g of sodium alginate, 5g of polyvinyl alcohol and 1.5g of p-toluenesulfonic acid into 100mL of deionized water, mix well, heat in a constant temperature water bath at 80°C to dissolve completely, cool to about 45°C, and use a constant temperature The flow pump drips it into 100mL of calcium chloride solution with a mass percentage of 1.5% at a speed of 60rpm and continuously stirs at a slow speed. After the calcium chloride solution is dripped, stop stirring, and place it at 4°C for crosslinking for 24 hours. After that, it was taken out and filtered to obtain immobilized p-toluenesulfonic acid.
[0024] 2) Esterification reaction
[0025] Add 308g of n-butanol, 200g of citric acid, and the immobilized p-toluenesulfonic acid obtained in step 1) into a 500mL three-neck flask equipped with electromagnetic stirring, a thermometer, a reflux condenser, and a water separator, and heat and reflux at 130°C. ...
Embodiment 2-4
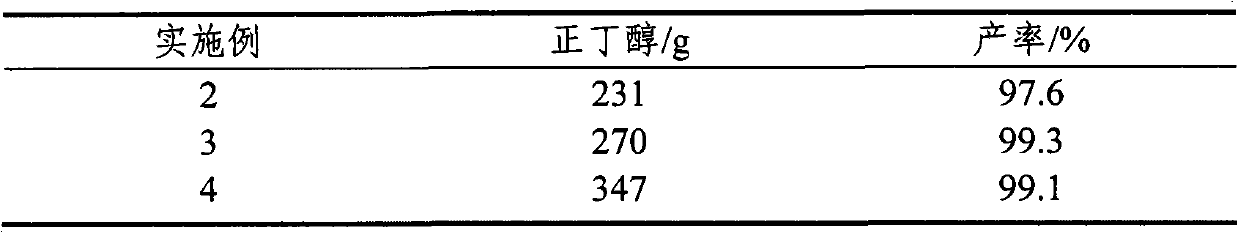
[0029] Only change n-butanol consumption, all the other are with embodiment 1. The results are shown in Table 1.
[0030] Table 1
[0031]
Embodiment 5-7
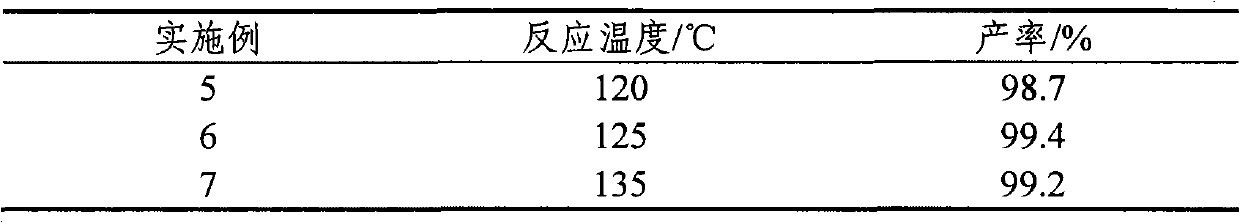
[0033] Only change the esterification reaction temperature, all the other are with embodiment 1. The results are shown in Table 2.
[0034] Table 2
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com