Method for synthesizing triethyl citrate
A technique for the synthesis of triethyl citrate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of three wastes, pollute the environment, and complicate post-processing, and achieve high product yields. , short reaction time and simplified post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of catalyst
[0029] Take 6g of diatomaceous earth and add it to 100mL of 4% titanium sulfate solution, and place it at 60℃ and stir for 2h, evaporate the water in vacuum, and the remaining solid will be dried at 100℃ for 24h. Calcination at ℃ for 6h.
[0030] 2) Esterification reaction
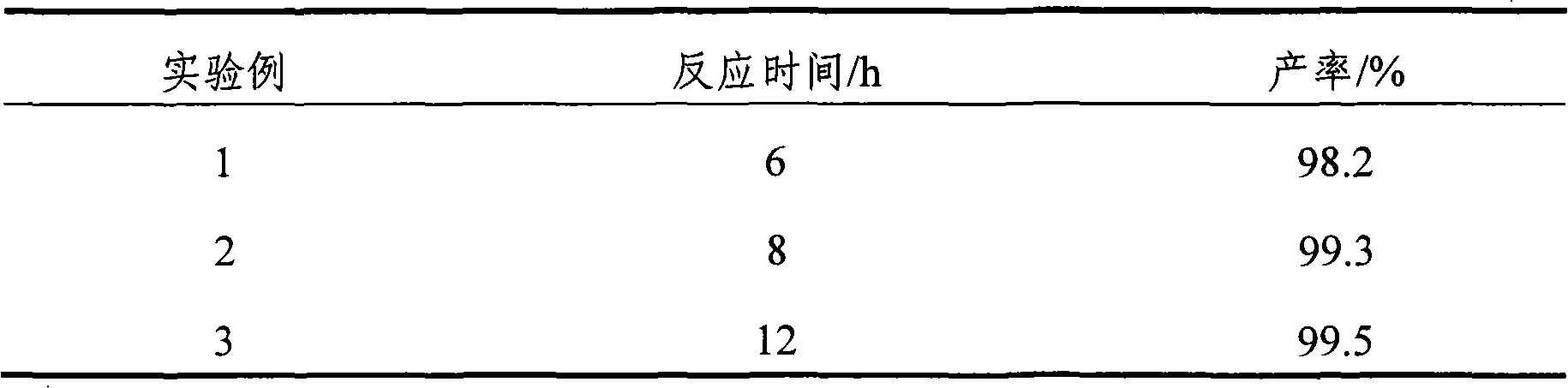
[0031] Add 192g of absolute ethanol (or 202g of recovered ethanol: about 95% by mass), 200g of citric acid and 3g of catalyst into a reactor equipped with electromagnetic stirring, thermometer and condenser. Heat and stir at 80℃, and collect the distillate as For the next batch of materials, anhydrous ethanol was added every 45 minutes during the reaction, and the volume of the alcohol was equal to the volume of the distillate. The acid value was measured at the beginning of the reaction and at the 10th hour, and the acid value was determined with reference to GB / T1668-2008.
[0032] 3) Catalyst recovery and product purification
[0033] Filter the above crude triethyl citrate,...
Embodiment 2
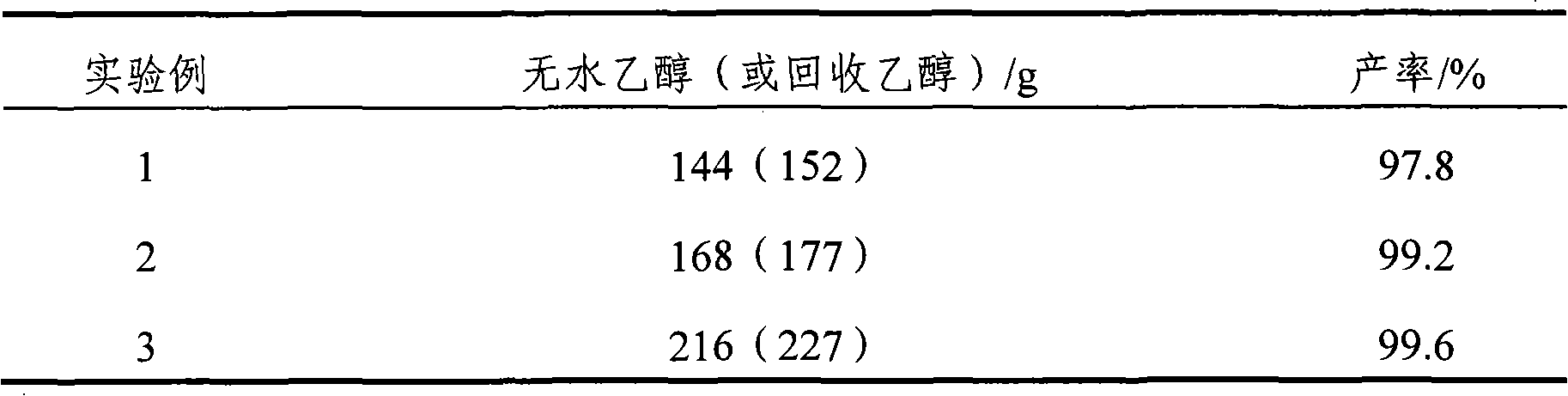
[0034] Example 2 The influence of the amount of absolute ethanol on the yield
[0035] The amount of anhydrous ethanol was 144g, 168g, and 216g, and other conditions were the same as in Example 1. The results of the effect of the amount of ethanol on the reaction are shown in Table 1.
[0036] Table 1 The effect of the amount of ethanol on the reaction yield
[0037]
[0038] Note: The mass fraction of recovered ethanol in Table 1 is about 95%
Embodiment 3
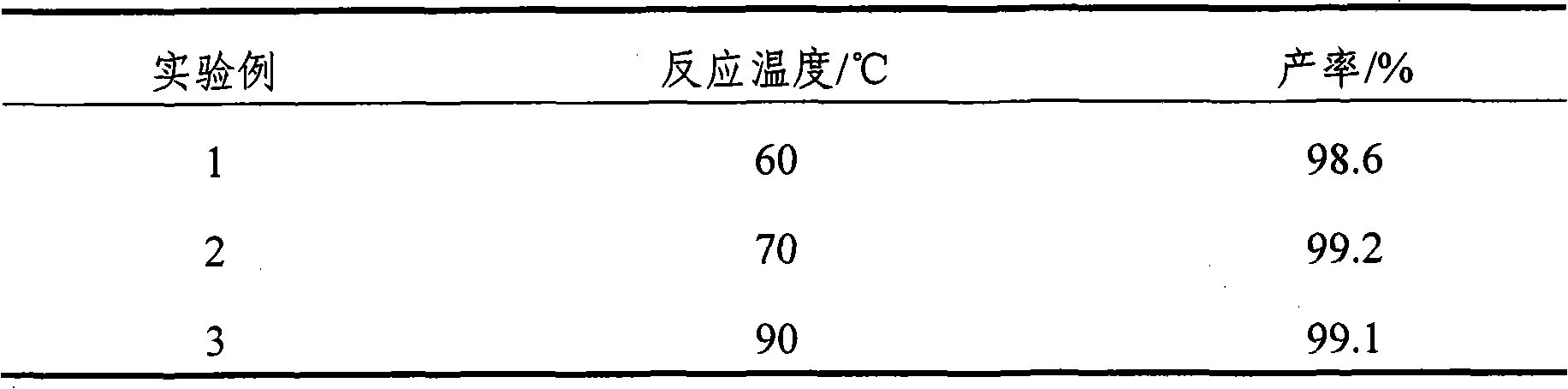
[0039] Example 3 Effect of the reaction temperature of the esterification reaction on the yield
[0040] The reaction temperature of the esterification reaction was 60° C., 70° C., and 90° C., and other conditions were the same as in Example 1. The effect of the reaction temperature on the reaction yield is shown in Table 2.
[0041] Table 2 Results of the effect of reaction temperature on reaction yield
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com