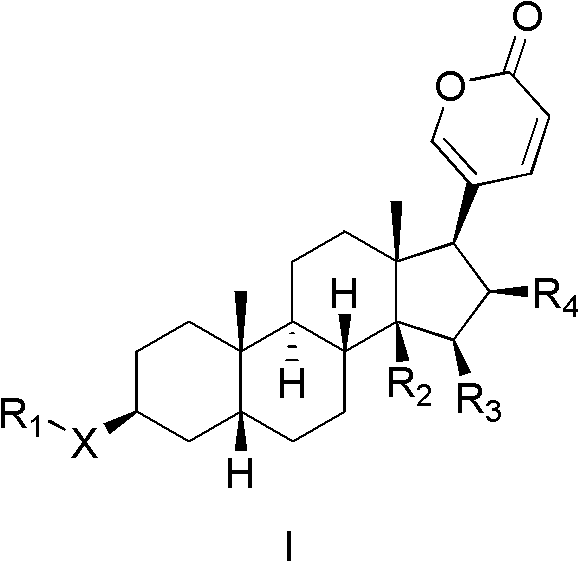
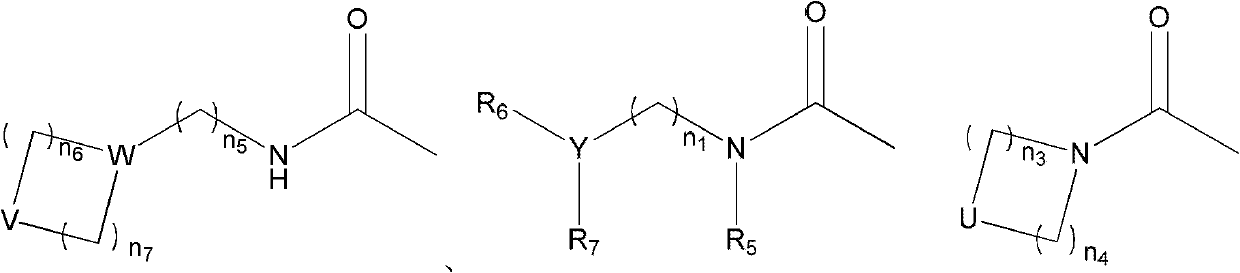
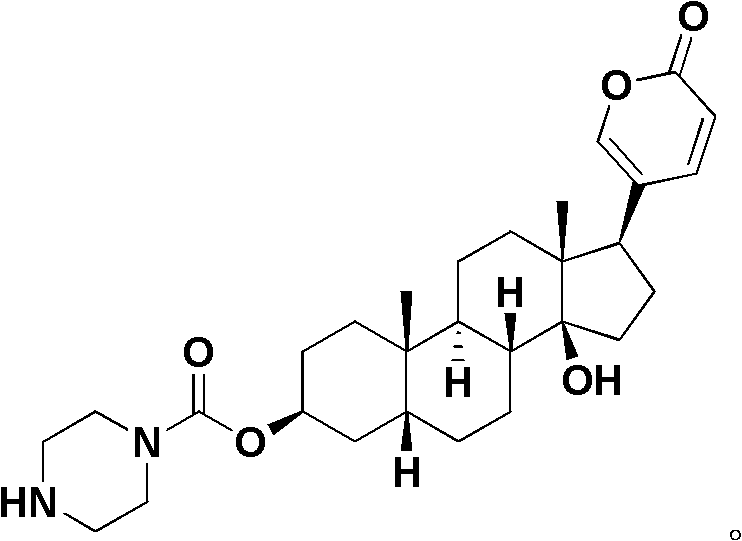
Bufogenin derivative and preparation method thereof, composition containing bufogenin derivative and applications thereof
A technology of bufagenin and derivatives, which can be applied in drug combinations, pharmaceutical formulations, steroids, etc., and can solve problems such as insufficient patient selection and unsatisfactory pharmacological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] The preparation of embodiment 1 compound II1B-01
[0109]
[0110] In a 50mL round-bottomed flask, dissolve p-nitrophenyl chloroformate (1.206g, 6mmol) in 10mL of anhydrous dichloromethane, add dry pyridine (0.67mL), a white precipitate appears immediately, and dropwise add A dichloromethane solution (10 mL) of bufalin (2 mmol) was stirred at room temperature for 6 hours, washed twice with water after the reaction was completed, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and subjected to silica gel column chromatography (90:10, Petroleum ether / acetone) to obtain intermediate A.
[0111] In a 10 mL round bottom flask, dissolve Intermediate A in 3 mL of dichloromethane, add triethylamine (35 μL), add N,N-dimethylethylenediamine (6 mmol), and stir at room temperature for 2 hours (following The reaction time of a similar reaction is determined by thin-layer chromatography detection), after the completion of the reaction, wash once with satur...
Embodiment 2
[0112] The preparation of embodiment 2 compound II1B-02
[0113]
[0114] The reaction operation is as in the preparation of compound II1B-01, the raw material is N, N-diethylethylenediamine instead of N, N-dimethylethylenediamine; silica gel column chromatography eluent: petroleum ether / acetone / ammonia water (50 :50:0.5), the yield was 78%. 1 H NMR (CDCl 3 , 300MHz) δ7.84(dd, 1H, J=9.6, 2.4Hz), 7.22(d, 1H, J=2.4Hz), 6.25(d, 1H, J=9.6Hz), 5.21(br s, 1H) , 3.23(d, 2H, J=5.4Hz), 2.55(m, 6H), 1.02(t, 6H, J=7.2Hz), 1.09-2.26(m, 22H), 0.94(s, 3H), 0.69( s, 3H); 13 C NMR (CDCl 3 , 75MHz) δ11.8, 11.8, 16.7, 21.5, 21.6, 23.9, 25.5, 26.6, 28.9, 29.9, 30.9, 32.9, 35.3, 36.0, 36.9, 38.7, 41.0, 42.5, 47.1, 47.1, 48.5, 51.4, 52.1 , 70.7, 85.5, 115.4, 122.9, 147.1, 148.7, 156.6, 162.6; ESI-MS (m / z) 529.5 [M+1] + .
Embodiment 3
[0115] The preparation of embodiment 3 compound II1B-03
[0116]
[0117] The reaction operation is as in the preparation of II1B-01, the raw material is N, N, N'-trimethylethylenediamine instead of N,N-dimethylethylenediamine; silica gel column chromatography eluent: petroleum ether / acetone / trimethylethylenediamine Ethylamine (60:40:0.5), 90% yield. 1 H NMR (CDCl 3 , 300MHz) δ7.83(dd, 1H, J=9.6, 2.4Hz), 7.22(d, 1H, J=2.4Hz), 6.24(d, 1H, J=9.6Hz), 4.99(brs, 1H), 3.36(t, 2H, J=7.2Hz), 3.36(s, 3H), 2.44(t, 2H, J=7.2Hz), 2.26(s, 6H), 1.20-2.20(m, 22H), 0.94(s , 3H), 0.69(s, 3H); 13 C NMR (CDCl 3 , 75MHz) δ16.7, 21.5, 21.6, 24.1, 25.5, 26.6, 28.9, 29.8, 30.9, 32.9, 34.6, 35.3, 36.0, 37.4, 41.0, 42.5, 47.1, 45.9, 48.5, 51.4, 57.0, 57.4, 71.2 , 85.4, 115.4, 122.9, 147.1, 148.7, 156.1, 162.6; ESI-MS (m / z) 515.3 [M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com