Soluble pharmaceutical forms of N,N'-diaminodiphenyl sulphone for optimum use in the treatment of various diseases
A diaminodiphenyl sulfone, disease technology, used in drug combinations, blood diseases, drug delivery and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Dissolution of N,N'-diaminodiphenylsulfone for administration as a solution for administration by any enteral or parenteral route.
[0032] 50 mg to 500 g of N,N'-diaminodiphenylsulfone are weighed and placed in a test tube. A 5-volume mixture containing 2.9 volumes of propylene glycol, 1.25 volumes of ethanol and 0.85 volumes of water was added. Shake the tube until the N,N'-diaminodiphenylsulfone is completely dissolved.
[0033] The obtained solution was transferred to a glass, sealed and sterilized by autoclaving.
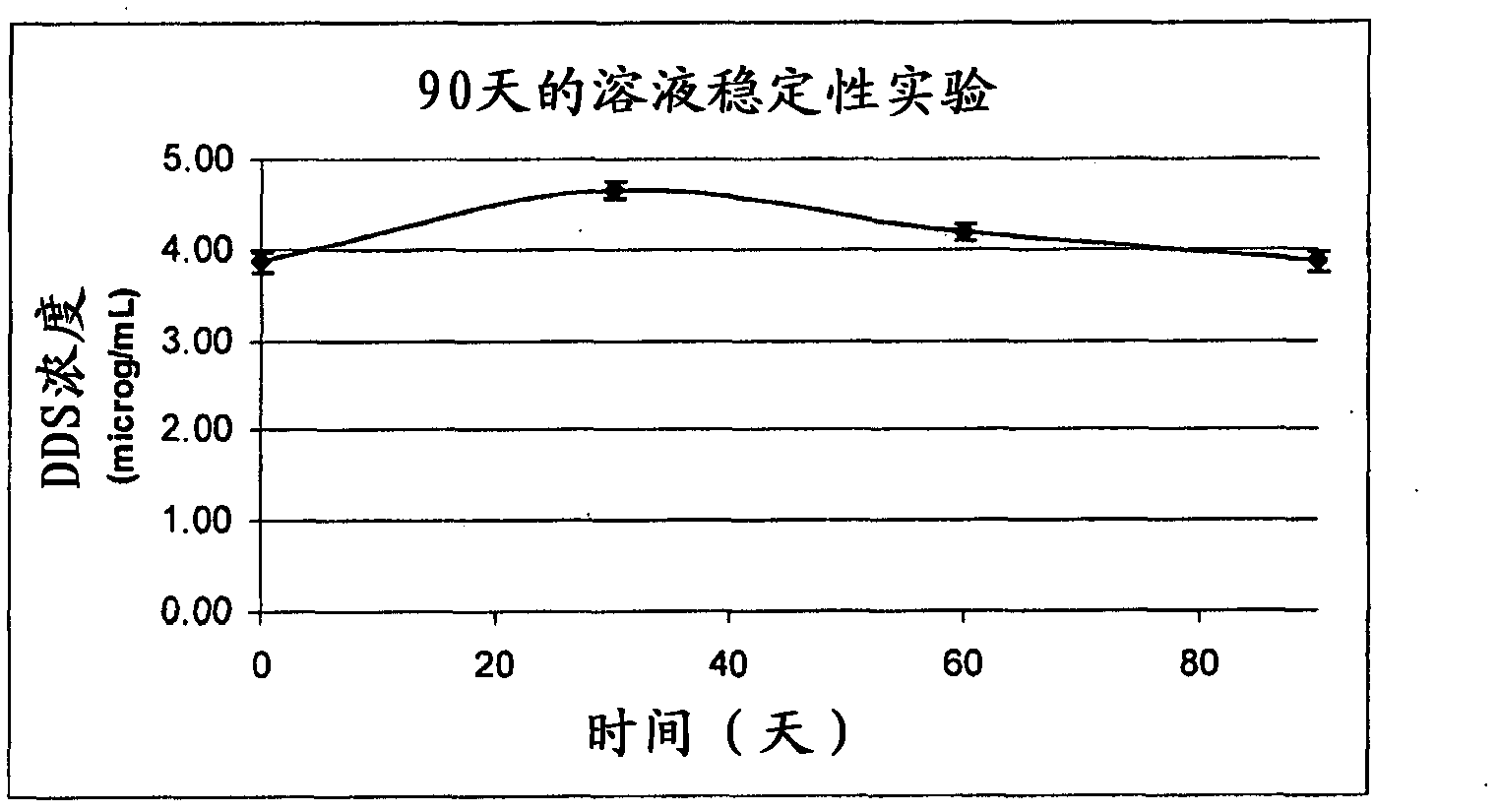
[0034] To determine whether N,N'-diaminodiphenylsulfone was completely dissolved and whether there was appreciable N,N'-diaminodiphenylsulfone degradation caused by the sterilization step, N,N'-diaminodiphenylsulfone was analyzed by high-resolution liquid chromatography by UV light detection. , N'-diaminodiphenylsulfone solution.
[0035] Assessing the effect of a soluble formulation of N,N'-diaminodiphenylsulfone on nervous system pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com