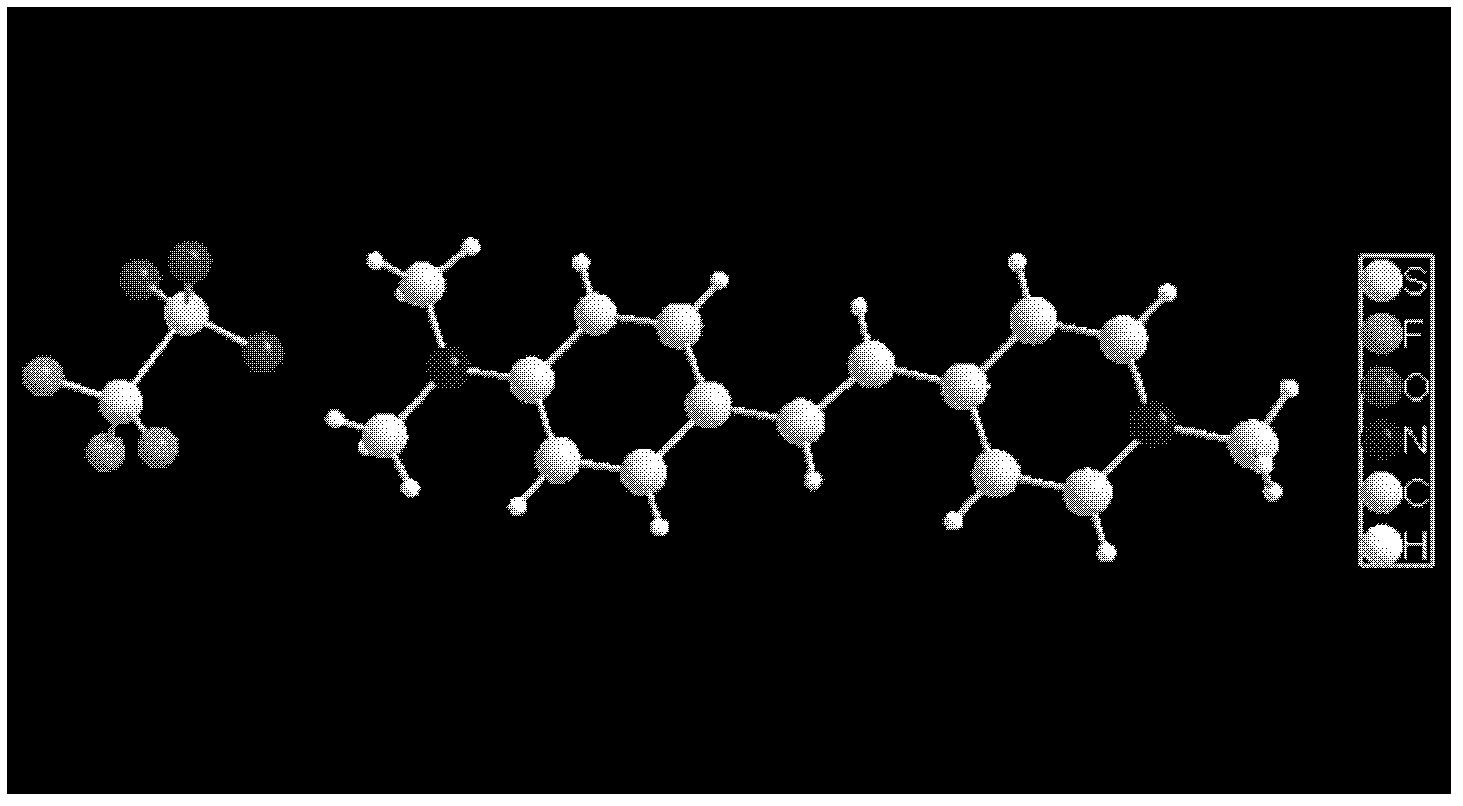
Organic three-order nonlinear optical material 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate and synthesizing method thereof
A technology of methylpyridine trifluorosulfonate and dimethylaminostyrene, which is applied in nonlinear optics, organic chemistry, sulfonate preparation, etc., can solve the complicated preparation process, long response time, limited research progress and other problems. Practical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0021] Implementation Case 1 Synthesis of 4-(4-dimethylaminostyryl)picoline iodide
[0022] Dissolve 0.01 mol of 4-methylpyridine, 0.01 mol of methyl iodide and 0.01 mol of 4-dimethylaminobenzaldehyde in 100-150 mL of methanol, stir and slowly raise the temperature to 65-70°C, add 3-5 mL of piperidine as The catalyst was refluxed for 10-12 hours, and the color of the solution gradually changed from light yellow to dark red. After the solution was cooled and crystallized, filtered and dried, 4-(4-dimethylaminostyryl)picoline iodide was obtained , and the yield was 84.2%.
[0023]
[0024] According to Example 1: 0.02 mol of 4-picoline, 0.02 mol of methyl iodide and 0.02 mol of 4-dimethylaminobenzaldehyde were dissolved in 100-150 mL of methanol, stirred and slowly heated to 65-70°C and refluxed for 48 h, the color of the solution gradually changed from light yellow to dark red. After the solution was crystallized by cooling, filtered and dried, 4-(4-dimethylaminostyryl)pic...
Embodiment example 2
[0026] Implementation Case 2 Synthesis of 4-(4-Dimethylaminostyryl)methylpyridine trifluorosulfonate
[0027] Under the condition of 50°C, react 0.01 mol 4-(4-dimethylaminostyryl)picoline iodide with 0.01-0.012 mol silver trifluorosulfonate in 50-75 mL methanol for about 2 h, filter After the precipitate was removed, the solution was allowed to stand and cooled to crystallize to obtain red needle-like or massive 4-(4-dimethylaminostyryl)picoline trifluorosulfonate crystals with a yield of 90.4%.
[0028] At room temperature, react 0.01 mol 4-(4-dimethylaminostyryl)picoline iodide with 0.01-0.012 mol silver trifluorosulfonate in 50-75 mL methanol for about 2 h, remove the precipitate by filtration, After the solution was allowed to stand and the solvent was slowly evaporated, red blocky 4-(4-dimethylaminostyryl)picoline trifluorosulfonate crystals were obtained with a yield of 84.7%.
[0029] At 50°C, 0.01 mol of 4-(4-dimethylaminostyryl)picoline iodide was dissolved in 75 m...
Embodiment example 3
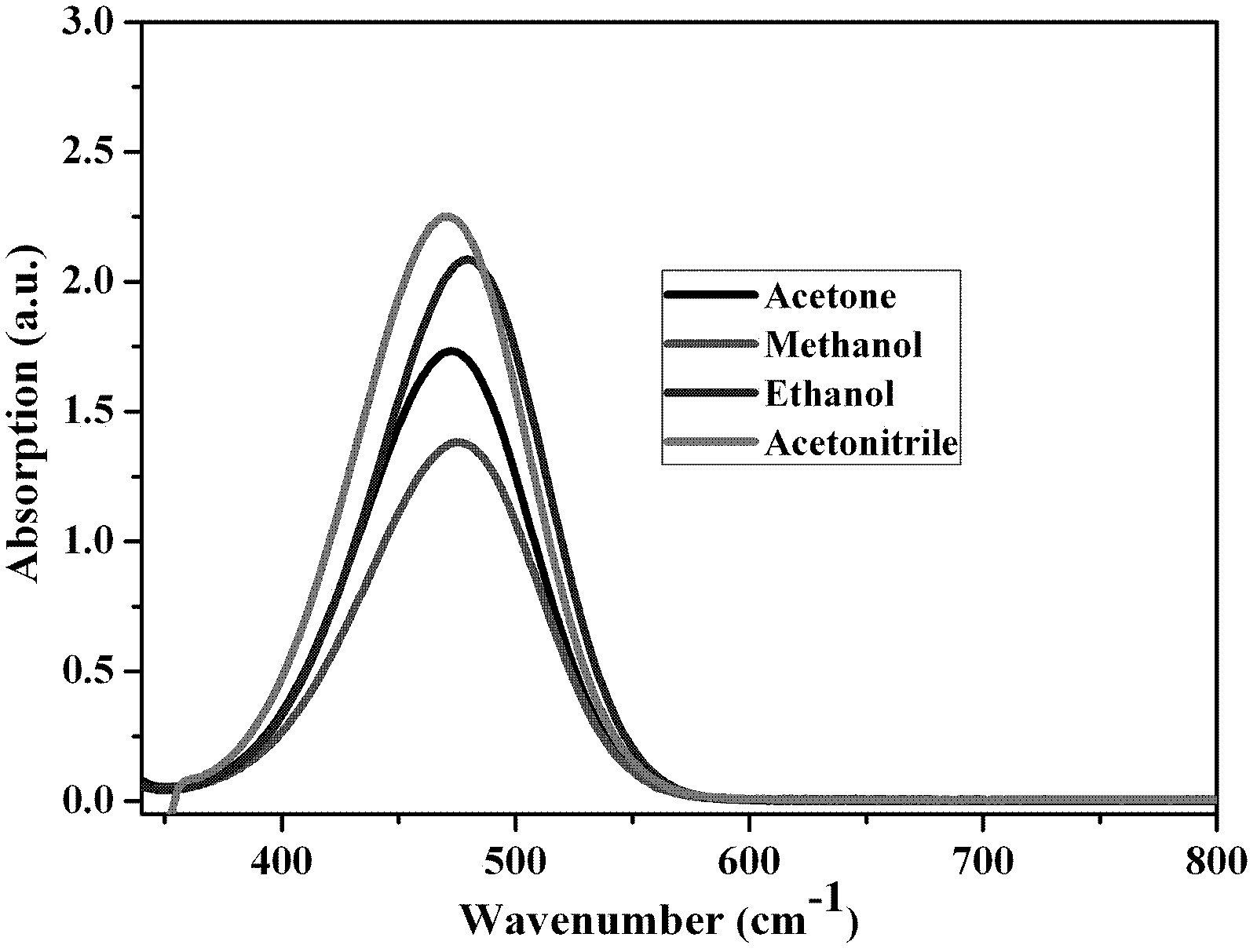
[0030] Implementation Case 3 The third-order nonlinear optical properties of 4-(4-dimethylaminostyryl)picoline trifluorosulfonate
[0031](1) Absorption spectrum of 4-(4-dimethylaminostyryl)picoline trifluorosulfonate
[0032] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was formulated into 2×10 -5 mol / L acetone solution, measure its absorption spectrum in the range of 350-800 nm.
[0033] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was formulated into 2×10 -5 mol / L methanol solution, and measure its absorption spectrum in the range of 350-800 nm.
[0034] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was formulated into 2×10 -5 mol / L ethanol solution, and measure its absorption spectrum in the range of 350-800 nm.
[0035] The 4-(4-dimethylaminostyryl) methylpyridine trifluorosulfonate prepared in the above examples was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com