Synthesizing method for N, N-double substitution-3-amino isoxazole-5-methanol compound
A technology for the synthesis of aminoisoxazoles and methods, which is applied in the field of synthesizing N,N-disubstituted-3-aminoisoxazole-5-methanol compounds, can solve the problems of long steps and harsh reaction conditions, and achieve mild reaction conditions , the reaction steps are simple, avoid the effect of catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
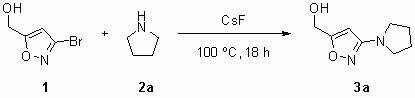
[0015] 3a Preparation of : Add 3-bromo-isoxazole-5-methanol (25 g, 140 mmol), cesium fluoride (42 g, 280 mmol) and pyrrolidine (20 mL ), when the addition is complete, seal the PTFE tank, and at 100 o C for 18 hours. After the reaction was completed, the jar was cooled to room temperature, the product was transferred to a 100 mL round-bottomed flask and spin-dried, then 100 mL of water was added to the flask to dissolve, extracted with dichloromethane (three times, 50 mL each), and combined The organic phase was dried with anhydrous sodium sulfate for one hour, filtered, concentrated, and separated by column chromatography (the volume ratio of developing solvent: petroleum ether to ethyl acetate was 2:1) to obtain the product 3-pyrrolidinyl-isoxazole-5- Methanol ( 3a ) 16.4 g, the yield was 70%. The product is a white solid.
[0016] 1 H NMR (400 MHz, d -DMSO) δ 5.90 (s, 1 H), 5.48 (t, J = 6.0 Hz, 1 H), 4.38 (d, J = 6.0 Hz, 2 H), 3.19-3.16 (m, 4 H), 1.8...
Embodiment 2
[0018]
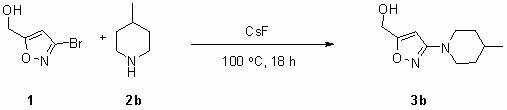
[0019] 3b Preparation: In a dry 100 mL tetrafluoro tank, add 3-bromo-isoxazole-5-methanol (15 g, 84 mmol), cesium fluoride (32 g, 210 mmol) and 4-methylpiperone in sequence Pyridine (15 mL), after the addition is complete, seal the tetrafluoro stuffy jar, at 100 o C for 36 hours. After the reaction was completed, the jar was cooled to room temperature, the product was transferred to a 100 mL round-bottomed flask and spin-dried, then 100 mL of water was added to the flask to dissolve, extracted with dichloromethane (three times, 50 mL each), and combined The organic phase. Dry with anhydrous sodium sulfate for one hour, filter, concentrate, and separate by column chromatography (developing solvent: petroleum ether to ethyl acetate volume ratio is 2:1) to obtain the product 3-(4-methylpiperidinyl)-isoxazole -5-methanol ( 3b ) 9.8 g, the yield was 60%. The product is a white solid.
[0020] 1 H NMR (400 MHz, d -DMSO) δ 6.11 (s,1 H), 5.50-5.47 (t, J = 6.0 Hz...
Embodiment 3
[0022]
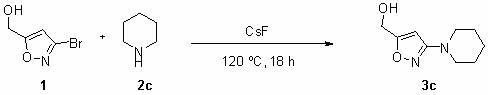
[0023] 3c Preparation of : Add 3-bromo-isoxazole-5-methanol (20 g, 112 mmol), cesium fluoride (17 g, 112 mmol) and piperidine (30 mL ), after the addition is complete, seal the PTFE tank, and at 120 o C for 72 hours. After the reaction was completed, the jar was cooled to room temperature, the product was transferred to a 100 mL round-bottomed flask and spin-dried, then 100 mL of water was added to the flask to dissolve, extracted with dichloromethane (three times, 50 mL each), and combined The organic phase. Dry with anhydrous sodium sulfate for one hour, filter, concentrate, and separate by column chromatography (developing solvent: sherwood oil to ethyl acetate volume ratio is 1:1) to obtain the product 3-piperidinyl-isoxazole-5-methanol ( 3c ) 16 g, the yield was 77 %. The product is a white solid.
[0024] 1 H NMR (400 MHz, d -DMSO) δ 6.14 (s, 1 H), 5.53-5.52 (t, J = 5.6 Hz, 1 H), 4.39-4.38 (d, J = 6.0 Hz, 2 H), 3.15-3.14 (m, 4 H), 1.54-1.53 (m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com