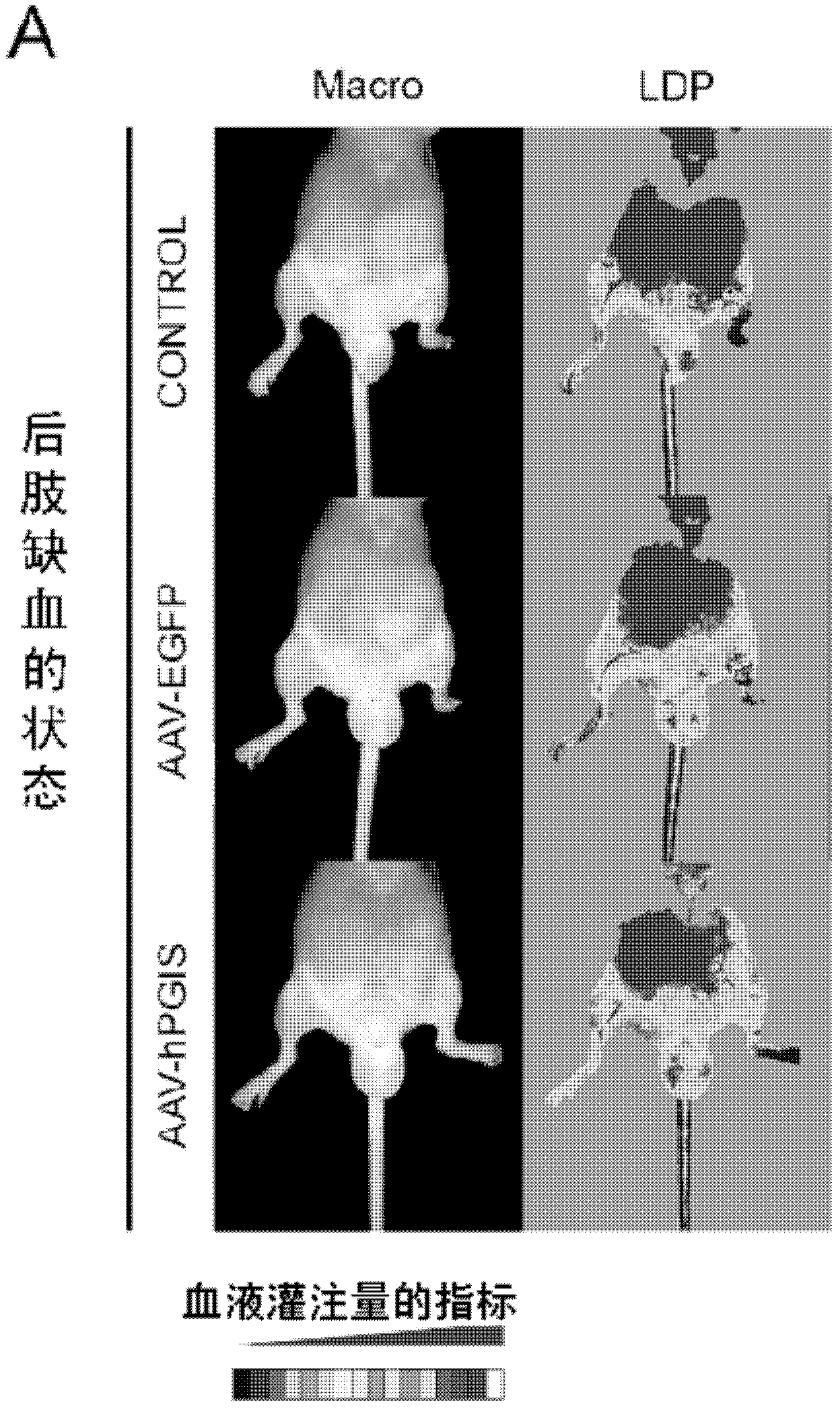
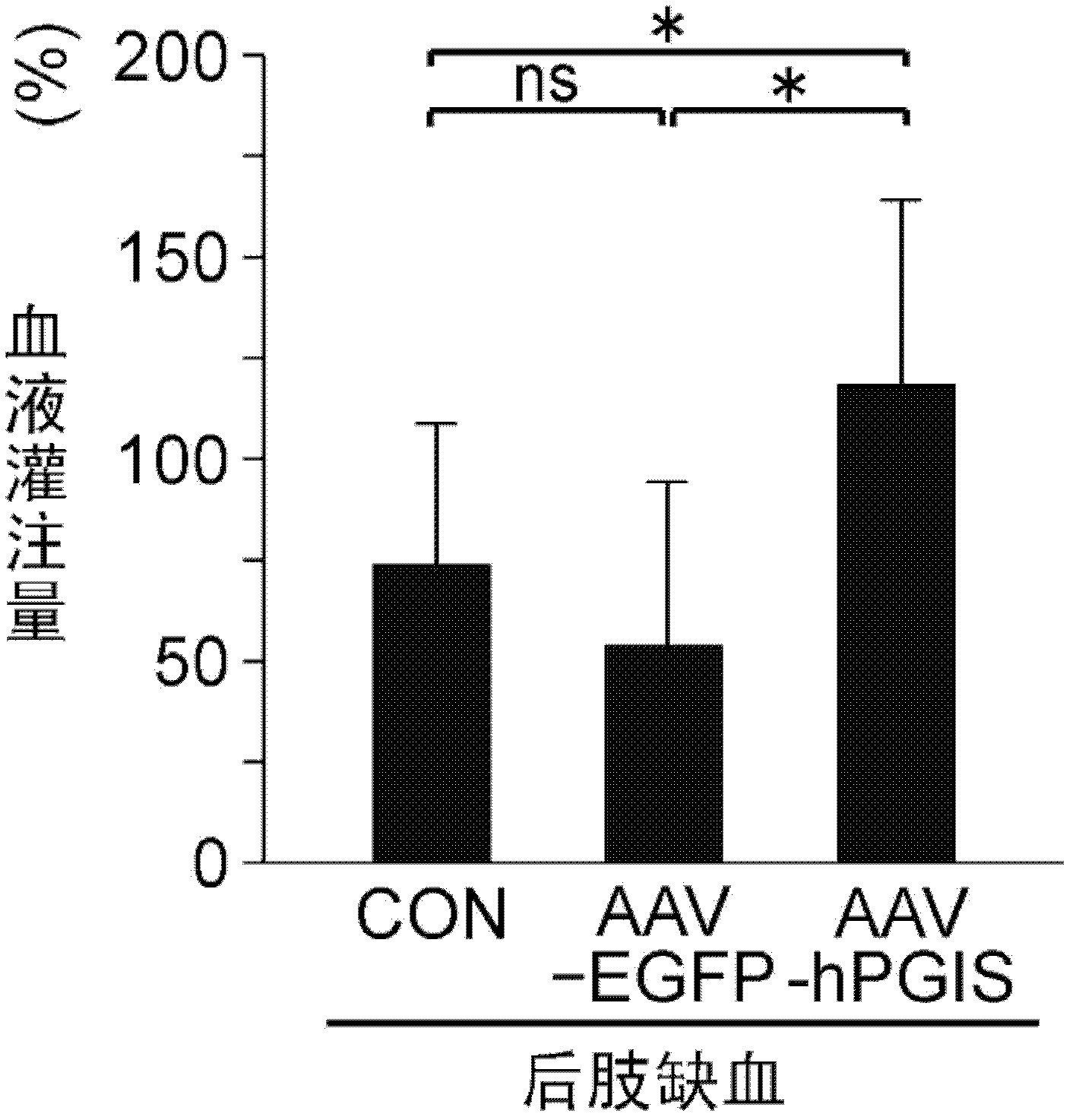
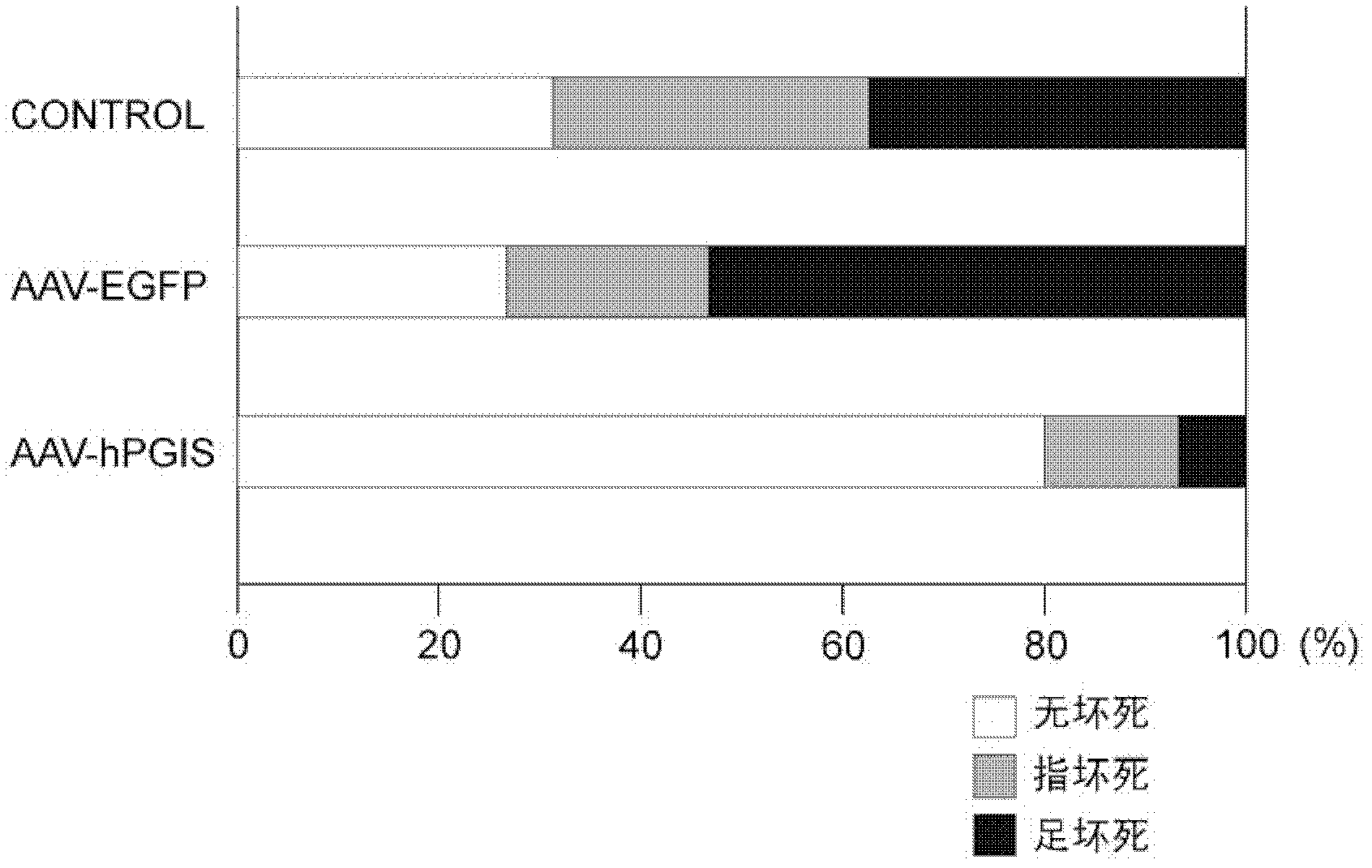
Drug composition for angiogenesis therapy
A pharmaceutical composition and angiogenesis technology, which can be used in drug combinations, microorganisms, pharmaceutical formulations, etc., and can solve problems such as limited effects of drug therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0068] Hereinafter, the present invention will be specifically explained through examples, but the present invention is not limited by these examples at all.
[0069] Construction of plasmid and adeno-associated virus (AAV) vector
[0070] The construction method of the human PGIS expression vector is briefly explained: the restriction endonuclease HindIII / BamHI fragment of the full-length human PGIS cDNA is smoothed, and the obtained fragment and the pUC-CAGGS expression plasmid are digested and smoothed with XhoI Location connection. In order to confirm that the pUC / PGIS construct encodes a biologically active PGIS protein, the pUC / PGIS vector was transfected into NIH3T3 cells, and the conversion of [14C]-PGH2 to 6-keto-[14C]-PGF1α was measured. The uninserted pUC-CAGGS vector was used as a control vector. Next, the human PGIS gene was inserted into the AAV-CAG plasmid to construct the AAV-hPGIS vector. In addition, a highly active green fluorescent protein (AAV-EGFP) for sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com